Notch1 control of oligodendrocyte differentiation in the spinal cord
- PMID: 12186854
- PMCID: PMC2174019
- DOI: 10.1083/jcb.200202002
Notch1 control of oligodendrocyte differentiation in the spinal cord
Abstract
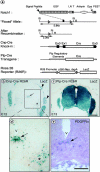
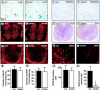
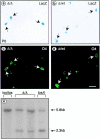
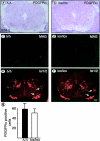
We have selectively inhibited Notch1 signaling in oligodendrocyte precursors (OPCs) using the Cre/loxP system in transgenic mice to investigate the role of Notch1 in oligodendrocyte (OL) development and differentiation. Early development of OPCs appeared normal in the spinal cord. However, at embryonic day 17.5, premature OL differentiation was observed and ectopic immature OLs were present in the gray matter. At birth, OL apoptosis was strongly increased in Notch1 mutant animals. Premature OL differentiation was also observed in the cerebrum, indicating that Notch1 is required for the correct spatial and temporal regulation of OL differentiation in various regions of the central nervous system. These findings establish a widespread function of Notch1 in the late steps of mammalian OPC development in vivo.
Figures


References
-
- Apelqvist, A., H. Li, L. Sommer, P. Beatus, D.J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signalling controls pancreatic cell differentiation. Nature. 400:877–881. - PubMed
-
- Artavanis-Tsakonas, S., K. Matsuno, and M.E. Fortini. 1995. Notch signaling. Science. 268:225–232. - PubMed
-
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. - PubMed
-
- Barres, B.A., and M.C. Raff. 1993. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 361:258–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

