REN: a novel, developmentally regulated gene that promotes neural cell differentiation
- PMID: 12186855
- PMCID: PMC2174014
- DOI: 10.1083/jcb.200202024
REN: a novel, developmentally regulated gene that promotes neural cell differentiation
Abstract
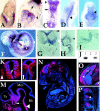
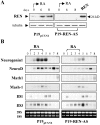
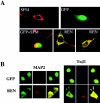
Expansion and fate choice of pluripotent stem cells along the neuroectodermal lineage is regulated by a number of signals, including EGF, retinoic acid, and NGF, which also control the proliferation and differentiation of central nervous system (CNS) and peripheral nervous system (PNS) neural progenitor cells. We report here the identification of a novel gene, REN, upregulated by neurogenic signals (retinoic acid, EGF, and NGF) in pluripotent embryonal stem (ES) cells and neural progenitor cell lines in association with neurotypic differentiation. Consistent with a role in neural promotion, REN overexpression induced neuronal differentiation as well as growth arrest and p27Kip1 expression in CNS and PNS neural progenitor cell lines, and its inhibition impaired retinoic acid induction of neurogenin-1 and NeuroD expression. REN expression is developmentally regulated, initially detected in the neural fold epithelium of the mouse embryo during gastrulation, and subsequently throughout the ventral neural tube, the outer layer of the ventricular encephalic neuroepithelium and in neural crest derivatives including dorsal root ganglia. We propose that REN represents a novel component of the neurogenic signaling cascade induced by retinoic acid, EGF, and NGF, and is both a marker and a regulator of neuronal differentiation.
Figures




References
-
- Bain, G., F.C. Mansergh, M.A. Wride, J.E. Hance, A. Isogawa, S.L. Rancourt, W.J. Ray, Y. Yoshimura, T. Tsuzuki, D.I. Gottlieb, and D.E. Rancourt. 2000. ES cell neural differentiation reveals a substantial number of novel ESTs. Funct. Integr. Genomics. 1:127–139. - PubMed
-
- Bang, O.S., E.K. Park, S.I. Yang, S.R. Lee, T.F. Franke, and S.S. Kang. 2001. Overexpression of Akt inhibits NGF-induced growth arrest and neuronal differentiation of PC12 cells. J. Cell Sci. 114:81–88. - PubMed
-
- Bardwell, V.J., and R. Treisman. 1994. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 8:1664–1677. - PubMed
-
- Bayer, S.A., and J. Altman. 1991. Neocortical Development. Raven, New York. 272 pp.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

