Novel PtdIns(3)P-binding protein Etf1 functions as an effector of the Vps34 PtdIns 3-kinase in autophagy
- PMID: 12186856
- PMCID: PMC2174002
- DOI: 10.1083/jcb.200112050
Novel PtdIns(3)P-binding protein Etf1 functions as an effector of the Vps34 PtdIns 3-kinase in autophagy
Abstract
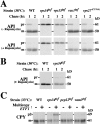
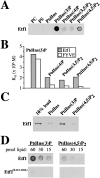
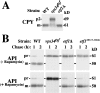
Autophagy is the process whereby cytoplasmic cargo (e.g., protein and organelles) are sequestered within a double membrane-enclosed transport vesicle and degraded after vesicle fusion with the vacuole/lysosome. Current evidence suggests that the Vps34 phosphatidylinositol 3-kinase is essential for macroautophagy, a starvation-induced autophagy pathway (Kihara et al., 2001). Here, we characterize a requirement for Vps34 in constitutive autophagy by the cytoplasm-to-vacuole targeting (Cvt) pathway. First, we show that transient disruption of phosphatidylinositol (PtdIns) 3-phosphate (PtdIns[3]P) synthesis through inactivation of temperature-sensitive Vps34 or its upstream activator, Vps15, blocks the Cvt and macroautophagy pathways. Yet, PtdIns(3)P-binding FYVE domain-containing proteins, which mediate carboxypeptidase Y (CPY) transport to the vacuole by the CPY pathway, do not account for the requirement of Vps34 in autophagy. Using a genetic selection designed to isolate PtdIns(3)P-binding effectors of Vps34, we identify Etf1, an uncharacterized type II transmembrane protein. Although Etf1 does not contain a known 3-phosphoinositide-binding domain (i.e., FYVE or Phox), we find that Etf1 interacts with PtdIns(3)P and that this interaction requires a basic amino acid motif (KKPAKK) within the cytosolic region of the protein. Moreover, deletion of ETF1 or mutation of the KKPAKK motif results in strong sorting defects in the Cvt pathway but not in macroautophagy or in CPY sorting. We propose that Vps34 regulates the CPY, Cvt, and macroautophagy pathways through distinct sets of PtdIns(3)P-binding effectors and that Vps34 promotes protein trafficking in the Cvt pathway through activation/localization of the effector protein Etf1.
Figures
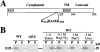




References
-
- Baukrowitz, T., and B. Fakler. 2000. KATP channels gated by intracellular nucleotides and phospholipids. Eur. J. Biochem. 267:5842–5848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

