Combination of drugs and drug-resistant reverse transcriptase results in a multiplicative increase of human immunodeficiency virus type 1 mutant frequencies
- PMID: 12186909
- PMCID: PMC136424
- DOI: 10.1128/jvi.76.18.9253-9259.2002
Combination of drugs and drug-resistant reverse transcriptase results in a multiplicative increase of human immunodeficiency virus type 1 mutant frequencies
Abstract
Replication of drug-resistant human immunodeficiency virus type 1 (HIV-1) in the presence of drug can lead to the failure of antiretroviral drug treatment. Drug failure is associated with the accumulation of drug resistance mutations. Previous studies have shown that 3'-azido-3'-deoxythymidine (AZT), (-)2',3'-dideoxy-3'-thiacytidine (3TC), and AZT-resistant HIV-1 reverse transcriptase (RT) can increase the virus in vivo mutation rate. In this study, the combined effects of drug-resistant RT and antiretroviral drugs on the HIV-1 mutant frequency were determined. In most cases, a multiplicative effect was observed with AZT-resistant or AZT/3TC dually resistant RT and several drugs (i.e., AZT, 3TC, hydroxyurea, and thymidine) and led to increases in the odds of recovering virus mutants to over 20 times that of the HIV-1 mutant frequency in the absence of drug or drug-resistance mutations. This observation indicates that HIV-1 can mutate at a significantly higher rate when drug-resistant virus replicates in the presence of drug. These increased mutant frequencies could have important implications for HIV-1 population dynamics and drug therapy regimens.
Figures
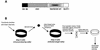
References
-
- Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908-15917. - PubMed
-
- Bangsberg, D. R., F. M. Hecht, E. D. Charlebois, A. R. Zolopa, M. Holodniy, L. Sheiner, J. D. Bamberger, M. A. Chesney, and A. Moss. 2000. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 14:357-366. - PubMed
-
- Biron, F., F. Lucht, D. Peyramond, A. Fresard, T. Vallet, F. Nugier, J. Grange, S. Malley, F. Hamedi-Sangsari, and J. Vila. 1996. Pilot clinical trial of the combination of hydroxyurea and didanosine in HIV-1 infected individuals. Antivir. Res. 29:111-113. - PubMed
-
- Coffin, J. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. - PubMed
-
- Cohen, A., J. Barankiewicz, H. M. Lederman, and E. W. Gelfand. 1983. Purine and pyrimidine metabolism in human T lymphocytes. Regulation of deoxyribonucleotide metabolism. J. Biol. Chem. 258:12334-12340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

