Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5
- PMID: 12186912
- PMCID: PMC136449
- DOI: 10.1128/jvi.76.18.9284-9297.2002
Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5
Abstract
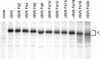
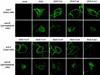
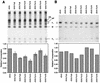
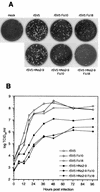
The efficient release of many enveloped viruses from cells involves the coalescence of viral components at sites of budding on the plasma membrane of infected cells. This coalescence is believed to require interactions between the cytoplasmic tails of surface glycoproteins and the matrix (M) protein. For the paramyxovirus simian virus 5 (SV5), the cytoplasmic tail of the hemagglutinin-neuraminidase (HN) protein has been shown previously to be important for normal virus budding. To investigate a role for the cytoplasmic tail of the fusion (F) protein in virus assembly and budding, we generated a series of F cytoplasmic tail-truncated recombinant viruses. Analysis of these viruses in tissue culture indicated that the cytoplasmic tail of the F protein was dispensable for normal virus replication and budding. To investigate further the requirements for assembly and budding of SV5, we generated two double-mutant recombinant viruses that lack 8 amino acids of the predicted 17-amino-acid HN protein cytoplasmic tail in combination with truncation of either 10 or 18 amino acids from the predicted 20-amino-acid F protein cytoplasmic tail. Both of the double mutant recombinant viruses displayed a replication defect in tissue culture and a budding defect, the extent of which was dependent on the length of the remaining F cytoplasmic tail. Taken together, this work and our earlier data on virus-like particle formation (A. P. Schmitt, G. P. Leser, D. L. Waning, and R. A. Lamb, J. Virol. 76:3953-3964, 2002) suggest a redundant role for the cytoplasmic tails of the HN and F proteins in virus assembly and budding.
Figures




Similar articles
-
Requirements for budding of paramyxovirus simian virus 5 virus-like particles.J Virol. 2002 Apr;76(8):3952-64. doi: 10.1128/jvi.76.8.3952-3964.2002. J Virol. 2002. PMID: 11907235 Free PMC article.
-
Involvement of the cytoplasmic domain of the hemagglutinin-neuraminidase protein in assembly of the paramyxovirus simian virus 5.J Virol. 1999 Oct;73(10):8703-12. doi: 10.1128/JVI.73.10.8703-8712.1999. J Virol. 1999. PMID: 10482624 Free PMC article.
-
Full conversion of the hemagglutinin-neuraminidase specificity of the parainfluenza virus 5 fusion protein by replacement of 21 amino acids in its head region with those of the simian virus 41 fusion protein.J Virol. 2013 Aug;87(15):8342-50. doi: 10.1128/JVI.03549-12. Epub 2013 May 22. J Virol. 2013. PMID: 23698295 Free PMC article.
-
Paramyxovirus membrane fusion: lessons from the F and HN atomic structures.Virology. 2006 Jan 5;344(1):30-7. doi: 10.1016/j.virol.2005.09.007. Virology. 2006. PMID: 16364733 Free PMC article. Review.
-
Escaping from the cell: assembly and budding of negative-strand RNA viruses.Curr Top Microbiol Immunol. 2004;283:145-96. doi: 10.1007/978-3-662-06099-5_5. Curr Top Microbiol Immunol. 2004. PMID: 15298170 Review.
Cited by
-
A role for caveolin 1 in assembly and budding of the paramyxovirus parainfluenza virus 5.J Virol. 2010 Oct;84(19):9749-59. doi: 10.1128/JVI.01079-10. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631121 Free PMC article.
-
Solution Structure, Self-Assembly, and Membrane Interactions of the Matrix Protein from Newcastle Disease Virus at Neutral and Acidic pH.J Virol. 2019 Mar 5;93(6):e01450-18. doi: 10.1128/JVI.01450-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30567981 Free PMC article.
-
Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity.Proc Natl Acad Sci U S A. 2004 Jun 29;101(26):9804-9. doi: 10.1073/pnas.0403492101. Epub 2004 Jun 21. Proc Natl Acad Sci U S A. 2004. PMID: 15210961 Free PMC article.
-
Role of the cytoplasmic domain of the Newcastle disease virus fusion protein in association with lipid rafts.J Virol. 2003 Dec;77(24):12968-79. doi: 10.1128/jvi.77.24.12968-12979.2003. J Virol. 2003. PMID: 14645553 Free PMC article.
-
Roles of the fusion and hemagglutinin-neuraminidase proteins in replication, tropism, and pathogenicity of avian paramyxoviruses.J Virol. 2011 Sep;85(17):8582-96. doi: 10.1128/JVI.00652-11. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680512 Free PMC article.
References
-
- Ali, A., and D. P. Nayak. 2000. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology 276:289-303. - PubMed
-
- Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

