The Epstein-Barr virus SM protein is functionally similar to ICP27 from herpes simplex virus in viral infections
- PMID: 12186924
- PMCID: PMC136475
- DOI: 10.1128/jvi.76.18.9420-9433.2002
The Epstein-Barr virus SM protein is functionally similar to ICP27 from herpes simplex virus in viral infections
Abstract
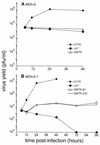
The herpes simplex virus type 1 (HSV-1) ICP27 protein is an essential RNA-binding protein that shuttles between the nucleus and cytoplasm to increase the cytoplasmic accumulation of viral late mRNAs. ICP27 homologs have been identified in each of the herpesvirus subfamilies, and accumulating evidence indicates that homologs from the gammaherpesvirus subfamily function similarly to ICP27. In particular, the Epstein-Barr virus (EBV) SM protein posttranscriptionally regulates gene expression, binds RNA in vitro and in vivo, and shuttles between the nucleus and cytoplasm. To determine if these two proteins function through a common mechanism, the ability of EBV SM to complement the growth defect of an HSV-1 ICP27-null virus was examined in a transient-expression assay. ICP27 stimulated the growth of the null mutant more efficiently than did SM, but the ability of SM to compensate for the ICP27 defects suggests conservation of common functions. To assay for complementation in the context of a viral infection, the growth properties of an HSV recombinant expressing SM in an ICP27-null background were analyzed. SM stimulated growth of the recombinant, although this growth was reduced by comparison to that of an ICP27-expressing virus. By contrast, an HSV recombinant expressing an SM mutant allele defective for transactivation activity and nucleocytoplasmic shuttling did not grow at all. These results suggest that SM and ICP27 may regulate gene expression through a common pathway that is evolutionarily conserved in herpesviruses.
Figures







References
-
- Bello, L. J., A. J. Davison, M. A. Glenn, A. Whitehouse, N. Rethmeier, T. F. Schulz, and J. Barklie Clements. 1999. The human herpesvirus-8 ORF 57 gene and its properties. J. Gen. Virol. 80:3207-3215. - PubMed
-
- Bogerd, H. P., G. L. Huckaby, Y. F. Ahmed, S. M. Hanly, and W. C. Greene. 1991. The type I human T-cell leukemia virus (HTLV-I) Rex trans-activator binds directly to the HTLV-I Rex and the type 1 human immunodeficiency virus Rev RNA response elements. Proc. Natl. Acad. Sci. USA 88:5704-5708. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

