Functional human CFTR produced by a stable minichromosome
- PMID: 12189175
- PMCID: PMC1084227
- DOI: 10.1093/embo-reports/kvf174
Functional human CFTR produced by a stable minichromosome
Abstract
Artificial chromosomes have been claimed to be the ideal vector for gene therapy, but their use has been hampered by an inability to produce stable and well designed molecules. We have used a structurally defined minichromosome to clone the human cystic fybrosis transmembrane conductance regulator (CFTR) locus. To guarantee the presence of the proper regulatory elements, we used the 320 kb yeast artificial chromosome (YAC) 37AB12 with the intact CFTR gene and upstream sequences. The resulting minichromosome was analyzed for the presence of the entire CFTR gene and for its functional activity by molecular and functional methods. We have identified clones showing the presence of both the transcript and the CFTR protein. Moreover, in the same clones, a chloride secretory response to cAMP was detected. Mitotic and molecular stability after prolonged growth without selection demonstrated that the constructs were stable. This is the first example of a structurally known minichromosome made to contain an active therapeutic gene.
Figures


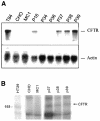
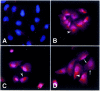

References
-
- Alton E. and Geddes, D. (1998) Cystic fibrosis clinical trials. Adv. Drug Deliv. Rev., 30, 205–217. - PubMed
-
- Barriere H., Poujeol, C., Tauc, M., Blasi, J.M., Counillon, L. and Poujeol, P. (2001) CFTR modulates programmed cell death by decreasing intracellular pH in Chinese hamster lung fibroblasts. Am. J. Physiol. Cell Physiol., 281, C810–C824. - PubMed
-
- Carine K., Solus, J., Waltzer, E., Manch-Citron, J., Hamkalo, B.A. and Scheffler, I.E. (1986) Chinese hamster cells with a minichromosome containing the centromeric region of human chromosome 1. Somat. Cell Mol. Genet., 12, 479–491. - PubMed
-
- Dechecchi M.C., Tamanini, A., Berton, G. and Cabrini, G. (1993) Protein kinase C activates chloride conductance in c127 cells stably expressing the cystic fibrosis gene. J. Biol. Chem., 268, 11321–11325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

