The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin
- PMID: 12189205
- PMCID: PMC129332
- DOI: 10.1073/pnas.182372299
The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin
Abstract
Suberoylanilide hydroxamic acid (SAHA) is a potent inhibitor of histone deacetylases (HDACs) that causes growth arrest, differentiation, and/or apoptosis of many tumor types in vitro and in vivo. SAHA is in clinical trials for the treatment of cancer. HDAC inhibitors induce the expression of less than 2% of genes in cultured cells. In this study we show that SAHA induces the expression of vitamin D-up-regulated protein 1/thioredoxin-binding protein-2 (TBP-2) in transformed cells. As the expression of TBP-2 mRNA is increased, the expression of a second gene, thioredoxin, is decreased. In transient transfection assays, HDAC inhibitors induce TBP-2 promoter constructs, and this induction requires an NF-Y binding site. We report here that TBP-2 expression is reduced in human primary breast and colon tumors compared with adjacent tissue. These results support a model in which the expression of a subset of genes (i.e., including TBP-2) is repressed in transformed cells, leading to a block in differentiation, and culture of transformed cells with SAHA causes re-expression of these genes, leading to induction of growth arrest, differentiation, and/or apoptosis.
Figures



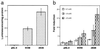

References
-
- Marks P A, Rifkind R A, Richon V M, Breslow R, Miller T, Kelly W K. Nat Rev Cancer. 2001;1:194–202. - PubMed
-
- Grunstein M. Nature (London) 1997;389:349–352. - PubMed
-
- Coffey D C, Kutko M C, Glick R D, Swendeman S L, Butler L, Rifkind R, Marks P A, Richon V M, LaQuaglia M P. Med Pediatr Oncol. 2000;35:577–581. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

