The Alzheimer's A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration
- PMID: 12189211
- PMCID: PMC129354
- DOI: 10.1073/pnas.192203399
The Alzheimer's A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration
Abstract
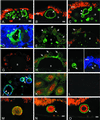
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in older individuals worldwide. The disease is characterized by abnormal extracellular deposits, known as drusen, that accumulate along the basal surface of the retinal pigmented epithelium. Although drusen deposition is common in older individuals, large numbers of drusen and/or extensive areas of confluent drusen represent a significant risk factor for AMD. Widespread drusen deposition is associated with retinal pigmented epithelial cell dysfunction and degeneration of the photoreceptor cells of the neural retina. Recent studies have shown that drusen contain a variety of immunomodulatory molecules, suggesting that the process of drusen formation involves local inflammatory events, including activation of the complement cascade. Similar observations in Alzheimer's disease (AD) have lead to the hypothesis that chronic localized inflammation is an important element of AD pathogenesis, with significant neurodegenerative consequences. Accordingly, the amyloid beta (A beta) peptide, a major constituent of neuritic plaques in AD, has been implicated as a primary activator of complement in AD. Here we show that A beta is associated with a substructural vesicular component within drusen. A beta colocalizes with activated complement components in these "amyloid vesicles," thereby identifying them as potential primary sites of complement activation. Thus, A beta deposition could be an important component of the local inflammatory events that contribute to atrophy of the retinal pigmented epithelium, drusen biogenesis, and the pathogenesis of AMD.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

