Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins
- PMID: 12189244
- PMCID: PMC150421
- DOI: 10.1172/JCI15780
Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins
Abstract
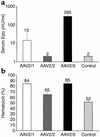
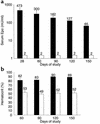
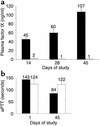
This study evaluates the use of vectors based on adeno-associated viruses (AAVs) to noninvasively deliver genes to airway epithelial cells as a means for achieving systemic administration of therapeutic proteins. We intranasally delivered AAV vectors to mice in which the same AAV2 genome encoding a cellular marker was packaged in capsids from AAV1, 2, or 5 (AAV2/1, AAV2/2, or AAV2/5, respectively). Gene expression levels achieved in both airways and alveoli were higher with AAV2/5 than with AAV2/1 and were undetectable with AAV2/2. The same set of vectors encoding a secreted therapeutic protein, erythropoietin (Epo), under the control of a lung-specific promoter (CC10) was intranasally delivered to mice, resulting in polycythemia with the highest levels of serum Epo obtained with AAV2/5 vectors. After a single intranasal administration of this vector, secretion of Epo was documented for 150 days. Similarly, intranasal administration of an AAV2/5-CC10-factor IX vector resulted in secretion of functional recombinant protein in the bloodstream of hemophiliac, factor IX-deficient mice. In addition, we demonstrate successful readministration of AAV2/5 to the lung 5 months after the first delivery of the same vector. In conclusion, we show that intranasal administration of AAV vectors results in efficient gene transfer to the lung only when the vector contains the AAV5 capsid and that this noninvasive route of administration results in sustained secretion of therapeutic proteins in the bloodstream.
Figures

Comment in
-
The lung as a metabolic factory for gene therapy.J Clin Invest. 2002 Aug;110(4):429-32. doi: 10.1172/JCI16443. J Clin Invest. 2002. PMID: 12189234 Free PMC article. No abstract available.
References
-
- Rosenfeld MA, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. - PubMed
-
- Kobinger GP, Weiner DJ, Yu QC, Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. - PubMed
-
- Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

