Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding
- PMID: 12189246
- PMCID: PMC150410
- DOI: 10.1172/JCI13847
Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding
Abstract
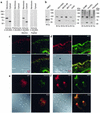
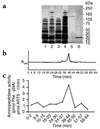
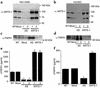
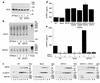
Proteolytic cleavage of TNF receptor 1 (TNFR1) generates soluble receptors that regulate TNF bioactivity. We hypothesized that the mechanism of TNFR1 shedding might involve interactions with regulatory ectoproteins. Using a yeast two-hybrid approach, we identified ARTS-1 (aminopeptidase regulator of TNFR1 shedding) as a type II integral membrane protein that binds to the TNFR1 extracellular domain. In vivo binding of membrane-associated ARTS-1 to TNFR1 was confirmed by coimmunoprecipitation experiments using human pulmonary epithelial and umbilical vein endothelial cells. A direct relationship exists between membrane-associated ARTS-1 protein levels and concordant changes in TNFR1 shedding. Cells overexpressing ARTS-1 demonstrated increased TNFR1 shedding and decreased membrane-associated TNFR1, while cells expressing antisense ARTS-1 mRNA demonstrated decreased membrane-associated ARTS-1, decreased TNFR1 shedding, and increased membrane-associated TNFR1. ARTS-1 neither bound to TNFR2 nor altered its shedding, suggesting specificity for TNFR1. Although a recombinant ARTS-1 protein demonstrated selective aminopeptidase activity toward nonpolar amino acids, multiple lines of negative evidence suggest that ARTS-1 does not possess TNFR1 sheddase activity. These data indicate that ARTS-1 is a multifunctional ectoprotein capable of binding to and promoting TNFR1 shedding. We propose that formation of a TNFR1-ARTS-1 molecular complex represents a novel mechanism by which TNFR1 shedding is regulated.
Figures


References
-
- Vilcek J, Lee TH. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991;266:7313–7316. - PubMed
-
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. - PubMed
-
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

