Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells
- PMID: 12192036
- PMCID: PMC135645
- DOI: 10.1128/MCB.22.18.6363-6374.2002
Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells
Abstract
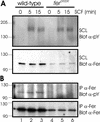
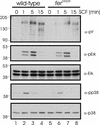
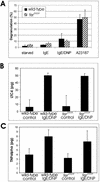
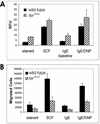
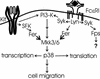
Mast cells play important roles in inflammation and immunity and express the high-affinity immunoglobulin E receptor (Fc epsilon RI) and the receptor protein-tyrosine kinase Kit. Aggregation of Fc epsilon RI via antigen binding elicits signals leading to the release of preformed inflammatory mediators as well as de novo-synthesized lipid mediators and cytokines and to elevated cell adhesion and migration. Here, we report that in mouse bone marrow-derived mast cells, Fer kinase is activated downstream of activated Fc epsilon RI and activated Kit receptor, and this activation is abolished in cells homozygous for a kinase-inactivating mutation in Fer (fer(DR/DR)). Interestingly, the highly related Fps/Fes kinase is also activated upon Fc epsilon RI aggregation. This report represents the first description of a common signaling pathway activating Fer and Fps/Fes. While Fer-deficient cells showed similar activation of the Erk mitogen-activated protein (MAP) kinases, p38 MAP kinase activation was less sustained than that in wild-type cells. Although no major defects were observed in degranulation, leukotriene biosynthesis, and cytokine secretion, Fer-deficient cells displayed increased adhesion and decreased motility upon activation of Fc epsilon RI and the Kit receptor. The restoration of Fer kinase activity in fer(DR/DR) mast cells resulted in prolonged p38 kinase activation and increased antigen-mediated cell migration of sensitized mast cells. Thus, Fer is required for maximal p38 kinase activation to promote the chemotaxis of activated mast cells. Further studies with mast cells derived from fps/fes-deficient mice will be required to provide insight into the role of Fps/Fes in mast cell activation.
Figures




Similar articles
-
Mitogen-activated protein kinase activation through Fc epsilon receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells.J Immunol. 1999 Feb 15;162(4):2087-94. J Immunol. 1999. PMID: 9973482
-
Fer and Fps/Fes participate in a Lyn-dependent pathway from FcepsilonRI to platelet-endothelial cell adhesion molecule 1 to limit mast cell activation.J Biol Chem. 2006 Jul 28;281(30):20949-20957. doi: 10.1074/jbc.M604252200. Epub 2006 May 26. J Biol Chem. 2006. PMID: 16731527
-
Fps/Fes protein-tyrosine kinase regulates mast cell adhesion and migration downstream of Kit and beta1 integrin receptors.Cell Signal. 2010 Mar;22(3):427-36. doi: 10.1016/j.cellsig.2009.10.014. Cell Signal. 2010. PMID: 19892014
-
FES/FER kinase signaling in hematopoietic cells and leukemias.Front Biosci (Landmark Ed). 2012 Jan 1;17(3):861-75. doi: 10.2741/3961. Front Biosci (Landmark Ed). 2012. PMID: 22201778 Review.
-
The tyrosine kinase network regulating mast cell activation.Immunol Rev. 2009 Mar;228(1):149-69. doi: 10.1111/j.1600-065X.2008.00742.x. Immunol Rev. 2009. PMID: 19290926 Free PMC article. Review.
Cited by
-
Electroporation-mediated delivery of the FER gene in the resolution of trauma-related fatal pneumonia.Gene Ther. 2016 Nov;23(11):785-796. doi: 10.1038/gt.2016.58. Epub 2016 Jul 22. Gene Ther. 2016. PMID: 27454317 Free PMC article.
-
Phosphoproteomics-mediated identification of Fer kinase as a target of mutant Shp2 in Noonan and LEOPARD syndrome.PLoS One. 2014 Sep 3;9(9):e106682. doi: 10.1371/journal.pone.0106682. eCollection 2014. PLoS One. 2014. PMID: 25184253 Free PMC article.
-
Contributions of F-BAR and SH2 domains of Fes protein tyrosine kinase for coupling to the FcepsilonRI pathway in mast cells.Mol Cell Biol. 2009 Jan;29(2):389-401. doi: 10.1128/MCB.00904-08. Epub 2008 Nov 10. Mol Cell Biol. 2009. PMID: 19001085 Free PMC article.
-
Electroporation-mediated delivery of FER gene enhances innate immune response and improves survival in a murine model of pneumonia.Gene Ther. 2018 Aug;25(5):359-375. doi: 10.1038/s41434-018-0022-y. Epub 2018 Jun 15. Gene Ther. 2018. PMID: 29907877 Free PMC article.
-
Fer and FerT Govern Mitochondrial Susceptibility to Metformin and Hypoxic Stress in Colon and Lung Carcinoma Cells.Cells. 2021 Jan 7;10(1):97. doi: 10.3390/cells10010097. Cells. 2021. PMID: 33430475 Free PMC article.
References
-
- Ahmed, S. T., and L. B. Ivashkiv. 2000. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J. Immunol. 165:5227-5237. - PubMed
-
- Asai, K., J. Kitaura, Y. Kawakami, N. Yamagata, M. Tsai, D. P. Carbone, F. T. Liu, S. J. Galli, and T. Kawakami. 2001. Regulation of mast cell survival by IgE. Immunity 14:791-800. - PubMed
-
- Aspenstrom, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479-487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
