Role of Ndt80, Sum1, and Swe1 as targets of the meiotic recombination checkpoint that control exit from pachytene and spore formation in Saccharomyces cerevisiae
- PMID: 12192042
- PMCID: PMC135635
- DOI: 10.1128/MCB.22.18.6430-6440.2002
Role of Ndt80, Sum1, and Swe1 as targets of the meiotic recombination checkpoint that control exit from pachytene and spore formation in Saccharomyces cerevisiae
Abstract
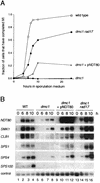
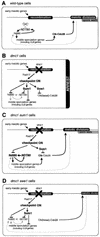
The meiotic recombination checkpoint, which is triggered by defects in recombination or chromosome synapsis, arrests sporulating cells of Saccharomyces cerevisiae at pachytene by preventing accumulation of active Clb-Cdc28. We compared the effects of manipulating the three known targets of the meiotic recombination checkpoint, NDT80, SWE1, and SUM1, in dmc1-arrested cells. Ndt80 is an activator of a set of middle sporulation-specific genes (MSGs), which includes CLB genes and genes involved in spore wall formation; Swe1 inhibits Clb-Cdc28 activity; and Sum1 is a repressor of NDT80 and some MSGs. Activation of the checkpoint leads to inhibition of Ndt80 activity and to stabilization of Swe1 and Sum1. Thus, dmc1-arrested cells fail to express MSGs, arrest at pachytene, and do not form spores. Our study shows that dmc1/dmc1 sum1/sum1 cells expressed MSGs prematurely and at high levels, entered the meiotic divisions efficiently, and in some cases formed asci containing mature spores. In contrast, dmc1/dmc1 swe1/swe1 cells expressed MSGs at a very low level, were inefficient and delayed in entry into the meiotic divisions, and never formed mature spores. We found that cells of dmc1/dmc1 sum1/sum1 ndt80/ndt80 and dmc1/dmc1 swe1/swe1 ndt80/ndt80 strains arrested at pachytene and that dmc1/dmc1 or dmc1/dmc1 swe1/swe1 cells overexpressing NDT80 were less efficient in bypassing checkpoint-mediated arrest than dmc1/dmc1 sum1/sum1 cells. Our results are consistent with previous suggestions that increased Clb-Cdc28 activity, caused by mutation of SWE1 or by an NDT80-dependent increase in CLB expression, allows dmc1/dmc1 cells to exit pachytene and that subsequent upregulation of Ndt80 activity by a feedback mechanism promotes entry into the meiotic divisions. Spore morphogenesis, however, requires efficient and timely activation of MSGs, which we speculate was achieved in dmc1/dmc1 sum1/sum1 cells by premature expression of NDT80.
Figures
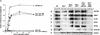


Similar articles
-
The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2012 Mar;76(1):1-15. doi: 10.1128/MMBR.05010-11. Microbiol Mol Biol Rev. 2012. PMID: 22390969 Free PMC article. Review.
-
NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae.Mol Cell Biol. 1998 Oct;18(10):5750-61. doi: 10.1128/MCB.18.10.5750. Mol Cell Biol. 1998. PMID: 9742092 Free PMC article.
-
The pachytene checkpoint in Saccharomyces cerevisiae requires the Sum1 transcriptional repressor.EMBO J. 2000 Dec 1;19(23):6489-97. doi: 10.1093/emboj/19.23.6489. EMBO J. 2000. PMID: 11101521 Free PMC article.
-
The Cdk1 and Ime2 protein kinases trigger exit from meiotic prophase in Saccharomyces cerevisiae by inhibiting the Sum1 transcriptional repressor.Mol Cell Biol. 2010 Jun;30(12):2996-3003. doi: 10.1128/MCB.01682-09. Epub 2010 Apr 12. Mol Cell Biol. 2010. PMID: 20385771 Free PMC article.
-
Meiosis: step-by-step through sporulation.Curr Biol. 1998 Jun 18;8(13):R461-3. doi: 10.1016/s0960-9822(98)70293-3. Curr Biol. 1998. PMID: 9651674 Review.
Cited by
-
Activity of phosphoforms and truncated versions of Ndt80, a checkpoint-regulated sporulation-specific transcription factor of Saccharomyces cerevisiae.Mol Genet Genomics. 2003 Dec;270(4):324-36. doi: 10.1007/s00438-003-0922-3. Epub 2003 Nov 7. Mol Genet Genomics. 2003. PMID: 14605875
-
The Ama1-directed anaphase-promoting complex regulates the Smk1 mitogen-activated protein kinase during meiosis in yeast.Genetics. 2005 Nov;171(3):901-11. doi: 10.1534/genetics.105.045567. Epub 2005 Aug 3. Genetics. 2005. PMID: 16079231 Free PMC article.
-
Dot1-dependent histone H3K79 methylation promotes activation of the Mek1 meiotic checkpoint effector kinase by regulating the Hop1 adaptor.PLoS Genet. 2013;9(1):e1003262. doi: 10.1371/journal.pgen.1003262. Epub 2013 Jan 31. PLoS Genet. 2013. PMID: 23382701 Free PMC article.
-
The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2012 Mar;76(1):1-15. doi: 10.1128/MMBR.05010-11. Microbiol Mol Biol Rev. 2012. PMID: 22390969 Free PMC article. Review.
-
Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis.Genes Dev. 2008 Oct 1;22(19):2627-32. doi: 10.1101/gad.1711408. Genes Dev. 2008. PMID: 18832066 Free PMC article.
References
-
- Amon, A., U. Surana, I. Muroff, and K. Nasmyth. 1992. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature 355:368-371. - PubMed
-
- Bailis, J. M., and G. S. Roeder. 2000. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell 101:211-221. - PubMed
-
- Bishop, D. K., D. Park, L. Xu, and N. Kleckner. 1992. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69:439-456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
