MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function
- PMID: 12192052
- PMCID: PMC135648
- DOI: 10.1128/MCB.22.18.6542-6552.2002
MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function
Abstract
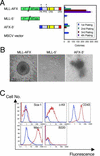
MLL-AFX is a fusion gene created by t(X;11) chromosomal translocations in a subset of acute leukemias of either myeloid or lymphoid derivation. It codes for a chimeric protein consisting of MLL fused to AFX, a forkhead transcription factor that normally regulates genes involved in apoptosis and cell cycle progression. We demonstrate here that forced expression of MLL-AFX enhances the self-renewal of hematopoietic progenitors in vitro and induces acute myeloid leukemias after long latencies in syngeneic recipient mice. MLL-AFX interacts with the transcriptional coactivator CBP, which is also a fusion partner for MLL in human leukemias. A potent minimal transactivation domain (CR3) at the C terminus of AFX mediates interactions with the KIX domain of CBP and is necessary for transformation of myeloid progenitors by MLL-AFX. However, CR3 alone is not sufficient, suggesting that simple acquisition of a transactivation domain per se does not activate the oncogenic potential of MLL. Rather, two conserved transcriptional effector domains (CR2 and CR3) of AFX are required for full oncogenicity of MLL-AFX and also endow it with the potential to competitively interfere with transcription and apoptosis mediated by wild-type forkhead proteins. Furthermore, a dominant-negative mutant of AFX containing CR2 and CR3 enhances the growth of myeloid progenitors in vitro, although considerably less effectively than does MLL-AFX. Taken together, these data suggest that recruitment of transcriptional cofactors utilized by forkhead proteins is a critical requirement for oncogenic action of MLL-AFX, which may impact both MLL- and forkhead-dependent transcriptional pathways.
Figures





Similar articles
-
Common mechanism for oncogenic activation of MLL by forkhead family proteins.Blood. 2003 Jan 15;101(2):633-9. doi: 10.1182/blood-2002-06-1785. Epub 2002 Aug 22. Blood. 2003. PMID: 12393557
-
The AF10 leucine zipper is required for leukemic transformation of myeloid progenitors by MLL-AF10.Blood. 2002 May 15;99(10):3780-5. doi: 10.1182/blood.v99.10.3780. Blood. 2002. PMID: 11986236
-
A carboxy-terminal domain of ELL is required and sufficient for immortalization of myeloid progenitors by MLL-ELL.Blood. 2000 Dec 1;96(12):3887-93. Blood. 2000. PMID: 11090074
-
Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins.Oncogene. 2001 Sep 10;20(40):5695-707. doi: 10.1038/sj.onc.1204639. Oncogene. 2001. PMID: 11607819 Review.
-
Structure of AF3p21, a new member of mixed lineage leukemia (MLL) fusion partner proteins-implication for MLL-induced leukemogenesis.Leuk Lymphoma. 2001 Aug;42(4):595-602. doi: 10.3109/10428190109099319. Leuk Lymphoma. 2001. PMID: 11697487 Review.
Cited by
-
FOXO transcription factors in cancer development and therapy.Cell Mol Life Sci. 2016 Mar;73(6):1159-72. doi: 10.1007/s00018-015-2112-y. Epub 2015 Dec 19. Cell Mol Life Sci. 2016. PMID: 26686861 Free PMC article. Review.
-
Identification and analysis of evolutionary selection pressures acting at the molecular level in five forkhead subfamilies.BMC Evol Biol. 2008 Sep 24;8:261. doi: 10.1186/1471-2148-8-261. BMC Evol Biol. 2008. PMID: 18816404 Free PMC article.
-
Critical role of retinoid/rexinoid signaling in mediating transformation and therapeutic response of NUP98-RARG leukemia.Leukemia. 2015 May;29(5):1153-62. doi: 10.1038/leu.2014.334. Epub 2014 Dec 16. Leukemia. 2015. PMID: 25510432
-
Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9.Genes Dev. 2003 Sep 15;17(18):2298-307. doi: 10.1101/gad.1111603. Epub 2003 Sep 2. Genes Dev. 2003. PMID: 12952893 Free PMC article.
-
Mechanisms of transcriptional regulation by MLL and its disruption in acute leukemia.Int J Hematol. 2008 Jan;87(1):10-8. doi: 10.1007/s12185-007-0009-8. Epub 2007 Dec 4. Int J Hematol. 2008. PMID: 18224408
References
-
- Anderson, M. J., C. S. Viars, S. Czekay, W. K. Cavenee, and K. C. Arden. 1998. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 47:187-199. - PubMed
-
- Ayton, P. M., and M. L. Cleary. 2001. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 20:5695-5707. - PubMed
-
- Barr, F. G., N. Galili, J. Holick, J. A. Biegel, G. Rovera, and B. S. Emanuel. 1993. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 3:113-117. - PubMed
-
- Bennicelli, J. L., S. Advani, B. W. Schafer, and F. G. Barr. 1999. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene 18:4348-4356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
