The carboxyl-terminal region of IkappaB kinase gamma (IKKgamma) is required for full IKK activation
- PMID: 12192055
- PMCID: PMC135629
- DOI: 10.1128/MCB.22.18.6573-6581.2002
The carboxyl-terminal region of IkappaB kinase gamma (IKKgamma) is required for full IKK activation
Abstract
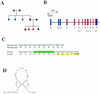
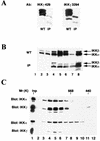
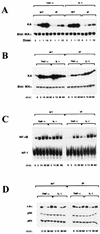
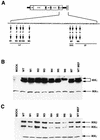
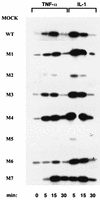
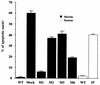
IkappaB kinase gamma (IKKgamma) (also known as NEMO, Fip-3, and IKKAP-1) is the essential regulatory component of the IKK complex; it is required for NF-kappaB activation by various stimuli, including tumor necrosis factor alpha (TNF-alpha), interleukin 1 (IL-1), phorbol esters, lipopolysaccharides, and double-stranded RNA. IKKgamma is encoded by an X-linked gene, deficiencies in which may result in two human genetic disorders, incontinentia pigmenti (IP) and hypohidrotic ectodermal dysplasia with severe immunodeficiency. Subsequent to the linkage of IKKgamma deficiency to IP, we biochemically characterized the effects of a mutation occurring in an IP-affected family on IKK activity and NF-kappaB signaling. This particular mutation results in premature termination, such that the variant IKKgamma protein lacks its putative C-terminal Zn finger and, due to decreased mRNA stability, is underexpressed. Correspondingly, IKK and NF-kappaB activation by TNF-alpha and, to a lesser extent, IL-1 are reduced. Mutagenesis of the C-terminal region of IKKgamma was performed in an attempt to define the role of the putative Zn finger and other potential functional motifs in this region. The mutants were expressed in IKKgamma-deficient murine embryonic fibroblasts (MEFs) at levels comparable to those of endogenous IKKgamma in wild-type MEFs and were able to associate with IKKalpha and IKKbeta. Substitution of two leucines within a C-terminal leucine zipper motif markedly reduced IKK activation by TNF-alpha and IL-1. Another point mutation resulting in a cysteine-to-serine substitution within the putative Zn finger motif affected IKK activation by TNF-alpha but not by IL-1. These results may explain why cells that express these or similar mutant alleles are sensitive to TNF-alpha-induced apoptosis despite being able to activate NF-kappaB in response to other stimuli.
Figures
Similar articles
-
The zinc finger domain of IKKγ (NEMO) protein in health and disease.J Cell Mol Med. 2010 Oct;14(10):2404-14. doi: 10.1111/j.1582-4934.2010.01054.x. J Cell Mol Med. 2010. PMID: 20345847 Free PMC article. Review.
-
The zinc finger mutation C417R of I-kappa B kinase gamma impairs lipopolysaccharide- and TNF-mediated NF-kappa B activation through inhibiting phosphorylation of the I-kappa B kinase beta activation loop.J Immunol. 2004 Feb 15;172(4):2446-52. doi: 10.4049/jimmunol.172.4.2446. J Immunol. 2004. PMID: 14764716
-
Tetrameric oligomerization of IkappaB kinase gamma (IKKgamma) is obligatory for IKK complex activity and NF-kappaB activation.Mol Cell Biol. 2003 Mar;23(6):2029-41. doi: 10.1128/MCB.23.6.2029-2041.2003. Mol Cell Biol. 2003. PMID: 12612076 Free PMC article.
-
A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha.J Biol Chem. 2003 Sep 26;278(39):37297-305. doi: 10.1074/jbc.M303389200. Epub 2003 Jul 16. J Biol Chem. 2003. PMID: 12867425
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
p120-catenin mediates inflammatory responses in the skin.Cell. 2006 Feb 10;124(3):631-44. doi: 10.1016/j.cell.2005.11.043. Cell. 2006. PMID: 16469707 Free PMC article.
-
Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways.EMBO J. 2007 Apr 4;26(7):1794-805. doi: 10.1038/sj.emboj.7601622. Epub 2007 Mar 15. EMBO J. 2007. PMID: 17363905 Free PMC article.
-
The zinc finger domain of IKKγ (NEMO) protein in health and disease.J Cell Mol Med. 2010 Oct;14(10):2404-14. doi: 10.1111/j.1582-4934.2010.01054.x. J Cell Mol Med. 2010. PMID: 20345847 Free PMC article. Review.
-
Interleukin-1-induced NF-kappaB activation is NEMO-dependent but does not require IKKbeta.J Biol Chem. 2007 Mar 23;282(12):8724-33. doi: 10.1074/jbc.M609613200. Epub 2007 Jan 23. J Biol Chem. 2007. PMID: 17244613 Free PMC article.
-
Intermolecular disulfide bond formation in the NEMO dimer requires Cys54 and Cys347.Biochem Biophys Res Commun. 2008 Feb 29;367(1):103-8. doi: 10.1016/j.bbrc.2007.12.123. Epub 2007 Dec 28. Biochem Biophys Res Commun. 2008. PMID: 18164680 Free PMC article.
References
-
- Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α induced cell death. Science 274:782-784. - PubMed
-
- Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-169. - PubMed
-
- Cao, Y., G. Bonizzi, T. Seagroves, Greten, F., R. Johnson, E. Schmidt, and M. Karin. 2001. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763-775. - PubMed
-
- Delhase, M., and M. Karin. 1999. The IκB kinase: a master regulator of NF-κB, innate immunity and epidermal differentiation. Cold Spring Harbor Symp. Quant. Biol. 64:491-503. - PubMed
-
- DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
