Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells
- PMID: 12192056
- PMCID: PMC135619
- DOI: 10.1128/MCB.22.18.6582-6591.2002
Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells
Abstract
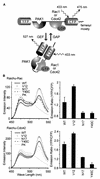
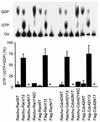
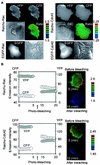
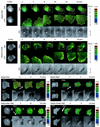
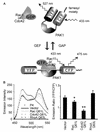
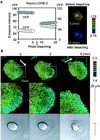
Rho family G proteins, including Rac and Cdc42, regulate a variety of cellular functions such as morphology, motility, and gene expression. We developed fluorescent resonance energy transfer-based probes which monitored the local balance between the activities of guanine nucleotide exchange factors and GTPase-activating proteins for Rac1 and Cdc42 at the membrane. These probes, named Raichu-Rac and Raichu-Cdc42, consisted of a Cdc42- and Rac-binding domain of Pak, Rac1 or Cdc42, a pair of green fluorescent protein mutants, and a CAAX box of Ki-Ras. With these probes, we video imaged the Rac and Cdc42 activities. In motile HT1080 cells, activities of both Rac and Cdc42 gradually increased toward the leading edge and decreased rapidly when cells changed direction. Under a higher magnification, we observed that Rac activity was highest immediately behind the leading edge, whereas Cdc42 activity was most prominent at the tip of the leading edge. Raichu-Rac and Raichu-Cdc42 were also applied to a rapid and simple assay for the analysis of putative guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) in living cells. Among six putative GEFs and GAPs, we identified KIAA0362/DBS as a GEF for Rac and Cdc42, KIAA1256 as a GEF for Cdc42, KIAA0053 as a GAP for Rac and Cdc42, and KIAA1204 as a GAP for Cdc42. In conclusion, use of these single-molecule probes to determine Rac and Cdc42 activity will accelerate the analysis of the spatiotemporal regulation of Rac and Cdc42 in a living cell.
Figures

Similar articles
-
RhoG signals in parallel with Rac1 and Cdc42.J Biol Chem. 2002 Dec 6;277(49):47810-7. doi: 10.1074/jbc.M203816200. Epub 2002 Oct 9. J Biol Chem. 2002. PMID: 12376551
-
Biosensors of DsRed as FRET partner with CFP or GFP for quantitatively imaging induced activation of Rac, Cdc42 in living cells.Mol Imaging Biol. 2011 Jun;13(3):424-431. doi: 10.1007/s11307-010-0381-2. Mol Imaging Biol. 2011. PMID: 20683671
-
TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG.J Cell Sci. 2000 Feb;113 ( Pt 4):729-39. doi: 10.1242/jcs.113.4.729. J Cell Sci. 2000. PMID: 10652265
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
Cited by
-
Essential role of class II phosphatidylinositol-3-kinase-C2α in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells.J Biol Chem. 2013 Jan 25;288(4):2325-39. doi: 10.1074/jbc.M112.409656. Epub 2012 Nov 28. J Biol Chem. 2013. PMID: 23192342 Free PMC article.
-
Rac1 and Cdc42 GTPases regulate shear stress-driven β-catenin signaling in osteoblasts.Biochem Biophys Res Commun. 2013 Apr 19;433(4):502-7. doi: 10.1016/j.bbrc.2013.03.020. Epub 2013 Mar 21. Biochem Biophys Res Commun. 2013. PMID: 23524265 Free PMC article.
-
The inositol 5-phosphatase SHIP2 is an effector of RhoA and is involved in cell polarity and migration.Mol Biol Cell. 2012 Jul;23(13):2593-604. doi: 10.1091/mbc.E11-11-0958. Epub 2012 May 16. Mol Biol Cell. 2012. PMID: 22593208 Free PMC article.
-
KCC2 regulates actin dynamics in dendritic spines via interaction with β-PIX.J Cell Biol. 2015 Jun 8;209(5):671-86. doi: 10.1083/jcb.201411008. J Cell Biol. 2015. PMID: 26056138 Free PMC article.
-
Anti-cancer potential of persimmon (Diospyros kaki) leaves via the PDGFR-Rac-JNK pathway.Sci Rep. 2020 Oct 22;10(1):18119. doi: 10.1038/s41598-020-75140-3. Sci Rep. 2020. PMID: 33093618 Free PMC article.
References
-
- Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. - PubMed
-
- Chamberlain, C. E., V. S. Kraynov, and K. M. Hahn. 2000. Imaging spatiotemporal dynamics of Rac activation in vivo with FLAIR. Methods Enzymol. 325:389-400. - PubMed
-
- Crespo, P., K. E. Schuebel, A. A. Ostrom, J. S. Gutkind, and X. R. Bustelo. 1997. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 385:169-172. - PubMed
-
- Day, R. N. 1998. Visualization of Pit-1 transcription factor interactions in the living cell nucleus by fluorescence resonance energy transfer microscopy. Mol. Endocrinol. 12:1410-1419. - PubMed
-
- del Pozo, M. A., W. B. Kiosses, N. B. Alderson, N. Meller, K. M. Hahn, and M. A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232-239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
