Interdomain engineered disulfide bond permitting elucidation of mechanisms of inactivation of coagulation factor Va by activated protein C
- PMID: 12192065
- PMCID: PMC2373598
- DOI: 10.1110/ps.0210002
Interdomain engineered disulfide bond permitting elucidation of mechanisms of inactivation of coagulation factor Va by activated protein C
Abstract
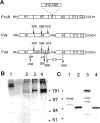
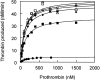
Procoagulant factor Va (FVa) is inactivated via limited proteolysis at three Arg residues in the A2 domain by the anticoagulant serine protease, activated protein C (APC). Cleavage by APC at Arg306 in FVa causes dissociation of the A2 domain from the heterotrimeric A1:A2:A3 structure and complete loss of procoagulant activity. To help distinguish inactivation mechanisms involving A2 domain dissociation from inactivation mechanisms involving unfavorable changes in factor Xa (FXa) affinity, we used our FVa homology model to engineer recombinant FVa mutants containing an interdomain disulfide bond (Cys609-Cys1691) between the A2 and A3 domains (A2-SS-A3 mutants) in addition to cleavage site mutations, Arg506Gln and Arg679Gln. SDS-PAGE analysis showed that the disulfide bond in A2-SS-A3 mutants prevented dissociation of the A2 domain. In the absence of A2 domain dissociation from the A1:A2:A3 trimer, APC cleavage at Arg306 alone caused a sevenfold decrease in affinity for FXa, whereas APC cleavages at Arg306, Arg506, and Arg679 caused a 70-fold decrease in affinity for FXa and a 10-fold decrease in the k(cat) of the prothrombinase complex for prothrombin without any effect on the apparent K(m) for prothrombin. Therefore, for FVa inactivation by APC, dissociation of the A2 domain may provide only a modest final step, whereas the critical events are the cleavages at Arg506 and Arg306, which effectively inactivate FVa before A2 dissociation can take place. Nonetheless, for FVa Leiden (Gln506-FVa) inactivation by APC, A2 domain dissociation may become mechanistically important, depending on the ambient FXa concentration.
Figures



Similar articles
-
Characterization of a factor Xa binding site on factor Va near the Arg-506 activated protein C cleavage site.J Biol Chem. 2007 Jul 27;282(30):21848-55. doi: 10.1074/jbc.M702192200. Epub 2007 Jun 6. J Biol Chem. 2007. PMID: 17553804
-
Prothrombin amino terminal region helps protect coagulation factor Va from proteolytic inactivation by activated protein C.Thromb Haemost. 2009 Jan;101(1):55-61. doi: 10.1160/th08-07-0491. Thromb Haemost. 2009. PMID: 19132189 Free PMC article.
-
Identification of surface epitopes of human coagulation factor Va that are important for interaction with activated protein C and heparin.J Biol Chem. 2008 Aug 15;283(33):22573-81. doi: 10.1074/jbc.M801724200. Epub 2008 Jun 2. J Biol Chem. 2008. PMID: 18519572
-
Factor Va-factor Xa interactions: molecular sites involved in enzyme:cofactor assembly.Scand J Clin Lab Invest Suppl. 2002;237:5-11. doi: 10.1080/003655102762377439. Scand J Clin Lab Invest Suppl. 2002. PMID: 12570161 Review.
-
Activated protein C resistance: molecular mechanisms.Thromb Haemost. 1995 Jul;74(1):444-8. Thromb Haemost. 1995. PMID: 8578503 Review.
Cited by
-
Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant.Blood. 2009 Jun 4;113(23):5970-8. doi: 10.1182/blood-2008-10-183327. Epub 2009 Feb 24. Blood. 2009. PMID: 19244160 Free PMC article.
-
An engineered factor Va prevents bleeding induced by direct-acting oral anticoagulants by different mechanisms.Blood Adv. 2020 Aug 11;4(15):3716-3727. doi: 10.1182/bloodadvances.2020001699. Blood Adv. 2020. PMID: 32777068 Free PMC article.
-
The tertiary structure and domain organization of coagulation factor VIII.Blood. 2008 Feb 1;111(3):1240-7. doi: 10.1182/blood-2007-08-109918. Epub 2007 Oct 26. Blood. 2008. PMID: 17965321 Free PMC article.
-
Progress in the understanding of the protein C anticoagulant pathway.Int J Hematol. 2004 Feb;79(2):109-16. doi: 10.1532/ijh97.03149. Int J Hematol. 2004. PMID: 15005336 Review.
-
Factor V is an anticoagulant cofactor for activated protein C during inactivation of factor Va.Pathophysiol Haemost Thromb. 2010;37(1):17-23. doi: 10.1159/000315141. Epub 2010 May 22. Pathophysiol Haemost Thromb. 2010. PMID: 20501981 Free PMC article.
References
-
- Bauer, K.A., Kass, B.L., ten Cate, H., Bednarek, M.A., Hawiger, J.J., and Rosenberg, R.D. 1989. Detection of factor X activation in humans. Blood 742007–2015. - PubMed
-
- Bernard, G.R., Vincent, J.L., Laterre, P.F., LaRosa, S.P., Dhainaut, J.F., Lopez-Rodriguez, A., Steingrub, J.S., Garber, G.E., Helterbrand, J.D., Ely, E.W., and Fisher, C.J., Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344699–709. - PubMed
-
- Brunger, A.T. 1992. X-PLOR Manual. Version 3.0. Yale University, New Haven.
-
- Creighton, T.E. 1984. Proteins. W.H. Freeman and Company, New York, pp. 152–157.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

