A structurally conserved water molecule in Rossmann dinucleotide-binding domains
- PMID: 12192068
- PMCID: PMC2373605
- DOI: 10.1110/ps.0213502
A structurally conserved water molecule in Rossmann dinucleotide-binding domains
Abstract
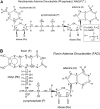
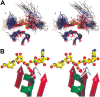
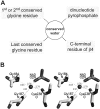
A computational comparison of 102 high-resolution (</=1.90 A) enzyme-dinucleotide (NAD, NADP, FAD) complexes was performed to investigate the role of solvent in dinucleotide recognition by Rossmann fold domains. The typical binding site contains about 9-12 water molecules, and about 30% of the hydrogen bonds between the protein and the dinucleotide are water mediated. Detailed inspection of the structures reveals a structurally conserved water molecule bridging dinucleotides with the well-known glycine-rich phosphate-binding loop. This water molecule displays a conserved hydrogen-bonding pattern. It forms hydrogen bonds to the dinucleotide pyrophosphate, two of the three conserved glycine residues of the phosphate-binding loop, and a residue at the C-terminus of strand four of the Rossmann fold. The conserved water molecule is also present in high-resolution structures of apo enzymes. However, the water molecule is not present in structures displaying significant deviations from the classic Rossmann fold motif, such as having nonstandard topology, containing a very short phosphate-binding loop, or having alpha-helix "A" oriented perpendicular to the beta-sheet. Thus, the conserved water molecule appears to be an inherent structural feature of the classic Rossmann dinucleotide-binding domain.
Figures

References
-
- Adolph, H.W., Zwart, P., Meijers, R., Hubatsch, I., Kiefer, M., Lamzin, V., and Cedergren-Zeppezauer, E. 2000. Structural basis for substrate specificity differences of horse liver alcohol dehydrogenase isozymes. Biochemistry 39 12885–12897. - PubMed
-
- Al-Karadaghi, S., Cedergren-Zeppezauer, E.S., Petratos, K., Hovmoeller, S., Terry, H., Dauter, Z., and Wilson, K.S. 1994. Refined crystal structure of liver alcohol dehydrogenase-NADH complex at 1.8 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 50 793–807. - PubMed
-
- Allaire, M., Li, Y., MacKenzie, R.E., and Cygler, M. 1998. The 3-D structure of a folate-dependent dehydrogenase/cyclohydrolase bifunctional enzyme at 1.5 Å resolution. Structure 6 173–182. - PubMed
-
- Aronov, A.M., Verlinde, C.L., Hol, W.G., and Gelb, M.H. 1998. Selective tight binding inhibitors of trypanosomal glyceraldehyde-3-phosphate dehydrogenase via structure-based drug design. J. Med. Chem. 41 4790–4799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

