Active site geometry of oxalate decarboxylase from Flammulina velutipes: Role of histidine-coordinated manganese in substrate recognition
- PMID: 12192069
- PMCID: PMC2373591
- DOI: 10.1110/ps.0206802
Active site geometry of oxalate decarboxylase from Flammulina velutipes: Role of histidine-coordinated manganese in substrate recognition
Abstract
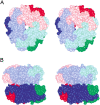
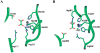
Oxalate decarboxylase (OXDC) from the wood-rotting fungus Flammulina velutipes, which catalyzes the conversion of oxalate to formic acid and CO(2) in a single-step reaction, is a duplicated double-domain germin family enzyme. It has agricultural as well as therapeutic importance. We reported earlier the purification and molecular cloning of OXDC. Knowledge-based modeling of the enzyme reveals a beta-barrel core in each of the two domains organized in the hexameric state. A cluster of three histidines suitably juxtaposed to coordinate a divalent metal ion exists in both the domains. Involvement of the two histidine clusters in the catalytic mechanism of the enzyme, possibly through coordination of a metal cofactor, has been hypothesized because all histidine knockout mutants showed total loss of decarboxylase activity. The atomic absorption spectroscopy analysis showed that OXDC contains Mn(2+) at up to 2.5 atoms per subunit. Docking of the oxalate in the active site indicates a similar electrostatic environment around the substrate-binding site in the two domains. We suggest that the histidine coordinated manganese is critical for substrate recognition and is directly involved in the catalysis of the enzyme.
Figures

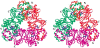


References
-
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215 403–410. - PubMed
-
- Banci, L., Bertini, I., Pozzo, L.D., Conte, R.D., and Tien, M. 1998. Monitoring the role of oxalate in manganese peroxidase. Biochemistry 37 9009–9015. - PubMed
-
- Baumlein, H., Braun, H., Kakhovskaya, I.A., and Shutov, A.D. 1995. Seed storage proteins of spermatophytes share a common ancestor with desiccation proteins of fungi. J. Mol. Evol. 41 1070–1075. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

