Plasticity in structure and interactions is critical for the action of indolicidin, an antibacterial peptide of innate immune origin
- PMID: 12192071
- PMCID: PMC2373604
- DOI: 10.1110/ps.0211602
Plasticity in structure and interactions is critical for the action of indolicidin, an antibacterial peptide of innate immune origin
Abstract
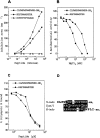
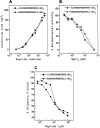
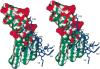
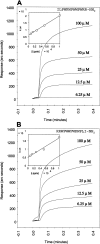
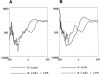
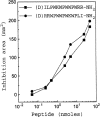
The comparative analysis of two cationic antibacterial peptides of the cathelicidin family-indolicidin and tritrypticin-enabled addressing the structural features critical for the mechanism of indolicidin activity. Functional behavior of retro-indolicidin was found to be identical to that of native indolicidin. It is apparent that the gross conformational propensities associated with retro-peptides resemble those of the native sequences, suggesting that native and retro-peptides can have similar structures. Both the native and the retro-indolicidin show identical affinities while binding to endotoxin, the initial event associated with the antibacterial activity of cationic peptide antibiotics. The indolicidin-endotoxin binding was modeled by docking the indolicidin molecule in the endotoxin structure. The conformational flexibility associated with the indolicidin residues, as well as that of the fatty acid chains of endotoxin combined with the relatively strong structural interactions, such as ionic and hydrophobic, provide the basis for the endotoxin-peptide recognition. Thus, the key feature of the recognition between the cationic antibacterial peptides and endotoxin is the plasticity of molecular interactions, which may have been designed for the purpose of maintaining activity against a broad range of organisms, a hallmark of primitive host defense.
Figures
Similar articles
-
Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA.Nucleic Acids Res. 2005 Jul 20;33(13):4053-64. doi: 10.1093/nar/gki725. Print 2005. Nucleic Acids Res. 2005. PMID: 16034027 Free PMC article.
-
Structure-activity relationship of indolicidin, a Trp-rich antibacterial peptide.J Pept Sci. 2010 Apr;16(4):171-7. doi: 10.1002/psc.1217. J Pept Sci. 2010. PMID: 20196123
-
Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles.Biochemistry. 2000 Dec 26;39(51):15765-74. Biochemistry. 2000. PMID: 11123901
-
Synergy among antibacterial peptides and between peptides and small-molecule antibiotics.Expert Rev Anti Infect Ther. 2010 Jun;8(6):703-16. doi: 10.1586/eri.10.38. Expert Rev Anti Infect Ther. 2010. PMID: 20521897 Review.
-
[Cationic antimicrobial peptides: from innate immunity study to drug development. Up date].Med Mal Infect. 2007 Apr;37(4):194-9. doi: 10.1016/j.medmal.2006.09.009. Epub 2007 Feb 15. Med Mal Infect. 2007. PMID: 17306486 Review. French.
Cited by
-
Structure-function analyses involving palindromic analogs of tritrypticin suggest autonomy of anti-endotoxin and antibacterial activities.Protein Sci. 2008 Mar;17(3):545-54. doi: 10.1110/ps.073145008. Epub 2008 Jan 24. Protein Sci. 2008. PMID: 18218719 Free PMC article.
-
Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA.Nucleic Acids Res. 2005 Jul 20;33(13):4053-64. doi: 10.1093/nar/gki725. Print 2005. Nucleic Acids Res. 2005. PMID: 16034027 Free PMC article.
-
Archetypal tryptophan-rich antimicrobial peptides: properties and applications.World J Microbiol Biotechnol. 2016 Feb;32(2):31. doi: 10.1007/s11274-015-1986-z. Epub 2016 Jan 9. World J Microbiol Biotechnol. 2016. PMID: 26748808 Review.
-
Molecular Interactions of the Antimicrobial Peptide Tritrpticin with Mixed Nanoaggregates: A Fluorescence Spectroscopy Study.Protein Pept Lett. 2025;32(2):152-160. doi: 10.2174/0109298665359223241226091327. Protein Pept Lett. 2025. PMID: 39878116
-
Reassessing the Host Defense Peptide Landscape.Front Chem. 2019 Feb 4;7:43. doi: 10.3389/fchem.2019.00043. eCollection 2019. Front Chem. 2019. PMID: 30778385 Free PMC article. Review.
References
-
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215 403–410. - PubMed
-
- Boman, H.G. 1991. Antibacterial peptides: Key components needed in immunity. Cell 65 205–207. - PubMed
-
- Casteels, P. and Tempst, P. 1994. Apidaecin-type peptide antibiotics function through a non-poreforming mechanism involving stereospecificity. Biochem. Biophys. Res. Commun. 199 339–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

