Crystal structures and enzymatic properties of three formyltransferases from archaea: environmental adaptation and evolutionary relationship
- PMID: 12192072
- PMCID: PMC2373594
- DOI: 10.1110/ps.0211002
Crystal structures and enzymatic properties of three formyltransferases from archaea: environmental adaptation and evolutionary relationship
Abstract
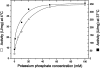
Formyltransferase catalyzes the reversible formation of formylmethanofuran from N(5)-formyltetrahydromethanopterin and methanofuran, a reaction involved in the C1 metabolism of methanogenic and sulfate-reducing archaea. The crystal structure of the homotetrameric enzyme from Methanopyrus kandleri (growth temperature optimum 98 degrees C) has recently been solved at 1.65 A resolution. We report here the crystal structures of the formyltransferase from Methanosarcina barkeri (growth temperature optimum 37 degrees C) and from Archaeoglobus fulgidus (growth temperature optimum 83 degrees C) at 1.9 A and 2.0 A resolution, respectively. Comparison of the structures of the three enzymes revealed very similar folds. The most striking difference found was the negative surface charge, which was -32 for the M. kandleri enzyme, only -8 for the M. barkeri enzyme, and -11 for the A. fulgidus enzyme. The hydrophobic surface fraction was 50% for the M. kandleri enzyme, 56% for the M. barkeri enzyme, and 57% for the A. fulgidus enzyme. These differences most likely reflect the adaptation of the enzyme to different cytoplasmic concentrations of potassium cyclic 2,3-diphosphoglycerate, which are very high in M. kandleri (>1 M) and relatively low in M. barkeri and A. fulgidus. Formyltransferase is in a monomer/dimer/tetramer equilibrium that is dependent on the salt concentration. Only the dimers and tetramers are active, and only the tetramers are thermostable. The enzyme from M. kandleri is a tetramer, which is active and thermostable only at high concentrations of potassium phosphate (>1 M) or potassium cyclic 2,3-diphosphoglycerate. Conversely, the enzyme from M. barkeri and A. fulgidus already showed these properties, activity and stability, at much lower concentrations of these strong salting-out salts.
Figures





Similar articles
-
Hyperthermophilic and salt-dependent formyltransferase from Methanopyrus kandleri.Biochem Soc Trans. 2004 Apr;32(Pt 2):269-72. doi: 10.1042/bst0320269. Biochem Soc Trans. 2004. PMID: 15046586 Review.
-
Comparison of three methyl-coenzyme M reductases from phylogenetically distant organisms: unusual amino acid modification, conservation and adaptation.J Mol Biol. 2000 Oct 20;303(2):329-44. doi: 10.1006/jmbi.2000.4136. J Mol Biol. 2000. PMID: 11023796
-
Primary structure and properties of the formyltransferase from the mesophilic Methanosarcina barkeri: comparison with the enzymes from thermophilic and hyperthermophilic methanogens.Arch Microbiol. 1996 Feb;165(2):97-105. doi: 10.1007/s002030050303. Arch Microbiol. 1996. PMID: 8593103
-
Salt dependence, kinetic properties and catalytic mechanism of N-formylmethanofuran:tetrahydromethanopterin formyltransferase from the extreme thermophile Methanopyrus kandleri.Eur J Biochem. 1992 Dec 15;210(3):971-81. doi: 10.1111/j.1432-1033.1992.tb17502.x. Eur J Biochem. 1992. PMID: 1483480
-
Heterodisulfide reductase from methanogenic archaea: a new catalytic role for an iron-sulfur cluster.Biol Chem. 2005 Oct;386(10):961-70. doi: 10.1515/BC.2005.112. Biol Chem. 2005. PMID: 16218868 Review.
Cited by
-
Elastin, a novel extracellular matrix protein adhering to mycobacterial antigen 85 complex.J Biol Chem. 2013 Feb 8;288(6):3886-96. doi: 10.1074/jbc.M112.415679. Epub 2012 Dec 17. J Biol Chem. 2013. PMID: 23250738 Free PMC article.
-
Comparative genomics reveals electron transfer and syntrophic mechanisms differentiating methanotrophic and methanogenic archaea.PLoS Biol. 2022 Jan 5;20(1):e3001508. doi: 10.1371/journal.pbio.3001508. eCollection 2022 Jan. PLoS Biol. 2022. PMID: 34986141 Free PMC article. Review.
-
Protein adaptations in archaeal extremophiles.Archaea. 2013;2013:373275. doi: 10.1155/2013/373275. Epub 2013 Sep 16. Archaea. 2013. PMID: 24151449 Free PMC article. Review.
-
Structural and functional analysis of the gpsA gene product of Archaeoglobus fulgidus: a glycerol-3-phosphate dehydrogenase with an unusual NADP+ preference.Protein Sci. 2004 Dec;13(12):3161-71. doi: 10.1110/ps.04980304. Protein Sci. 2004. PMID: 15557260 Free PMC article.
-
Denaturation of an extremely stable hyperthermophilic protein occurs via a dimeric intermediate.Extremophiles. 2007 Jan;11(1):179-89. doi: 10.1007/s00792-006-0030-5. Epub 2006 Oct 28. Extremophiles. 2007. PMID: 17072686
References
-
- Boone, D.R., Whitman, W.B., and Rouviére, P. 1993. Diversity and taxonomy of methanogens. In Methanogenesis (ed. J.G. Ferry), pp. 35–80. Chapman & Hall, New York.
-
- Breitung, J. and Thauer, R.K. 1990. Formylmethanofuran:tetrahydromethanopterin formyltransferase from Methanosarcina barkeri: Identification of N5-formyltetrahydromethanopterin as the product.FEBS Lett. 275 226–230. - PubMed
-
- Breitung, J., Börner, G., Scholz, S., Linder, D., Stetter, K.O., and Thauer, R.K. 1992. Salt dependence, kinetic properties and catalytic mechanism of N-formylmethano-furan:tetrahydromethanopterin formyltransferase from the extreme thermophile Methanopyrus kandleri.Eur. J. Biochem. 210 971–981. - PubMed
-
- Brünger, A., Adams, P.D., Clore, G.M., Delano, W.L., Gros, P., GrosseKunstleve, R., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography and NMR system: A new software suite for macromolecular structure determinations.Acta Crystallogr. D 54 905–921. - PubMed
-
- CCP4. 1994. The CCP4 Suite: Programs for protein crystallography.Acta Crystallogr. D 50 760–763. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

