The role of disulfide bond in the amyloidogenic state of beta(2)-microglobulin studied by heteronuclear NMR
- PMID: 12192077
- PMCID: PMC2373597
- DOI: 10.1110/ps.0213202
The role of disulfide bond in the amyloidogenic state of beta(2)-microglobulin studied by heteronuclear NMR
Abstract
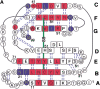
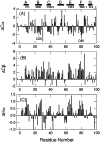
beta(2)-Microglobulin (beta2-m) is a major component of dialysis-related amyloid fibrils. Although recombinant beta2-m forms needle-like fibrils by in vitro extension reaction at pH 2.5, reduced beta2-m, in which the intrachain disulfide bond is reduced, cannot form typical fibrils. Instead, thinner and flexible filaments are formed, as shown by atomic force microscopy images. To clarify the role of the disulfide bond in amyloid fibril formation, we characterized the conformations of the oxidized (intact) and reduced forms of beta2-m in the acid-denatured state at pH 2.5, as well as the native state at pH 6.5, by heteronuclear NMR. [(1)H]-(15)N NOE at the regions between the two cysteine residues (Cys25-Cys80) revealed a marked difference in the pico- and nanosecond time scale dynamics between that the acid-denatured oxidized and reduced states, with the former showing reduced mobility. Intriguingly, the secondary chemical shifts, DeltaCalpha, DeltaCO, and DeltaHalpha, and (3)J(HNHalpha) coupling constants indicated that both the oxidized and reduced beta2-m at pH 2.5 have marginal alpha-helical propensity at regions close to the C-terminal cysteine, although it is a beta-sheet protein in the native state. The results suggest that the reduced mobility of the denatured state is an important factor for the amylodogenic potential of beta2-m, and that the marginal helical propensity at the C-terminal regions might play a role in modifying this potential.
Figures






References
-
- Alexandrescu, A. 2001. An NMR-based quenched hydrogen exchange investigation of model amyloid fibrils formed by cold shock protein A. Pac. Symp. Biocomput. 67–78. - PubMed
-
- Bjorkman, P.J., Saper, M.A., Samraoui, B., Bennett, W.S., Strominger, J.L., and Wiley, D.C. 1987. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329 506–512. - PubMed
-
- Brange, J., Andersen, L., Laursen, E.D., Meyn, G., and Rasmussen, E. 1997. Toward understanding insulin fibrillation. J. Pharm. Sci. 86 517–525. - PubMed
-
- Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C.M., and Stefani, M. 2002. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416 507–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

