Pheromone binding by polymorphic mouse major urinary proteins
- PMID: 12192080
- PMCID: PMC2373590
- DOI: 10.1110/ps.0204202
Pheromone binding by polymorphic mouse major urinary proteins
Erratum in
- Protein Sci. 2004 Jan;13(1):306
Abstract
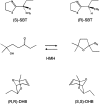
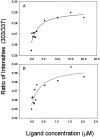
Mouse major urinary proteins (MUPs) have been proposed to play a role in regulating the release and capture of pheromones. Here, we report affinity measurements of five recombinant urinary MUP isoforms (MUPs-I, II, VII, VIII, and IX) and one recombinant nasal isoform (MUP-IV) for each of three pheromonal ligands, (+/-)-2-sec-butyl-4,5-dihydrothiazole (SBT), 6-hydroxy-6-methyl-3-heptanone (HMH), and (+/-)dehydro-exo-brevicomin (DHB). Dissociation constants for all MUP-pheromone pairs were determined by isothermal titration calorimetry, and data for SBT were corroborated by measurements of intrinsic protein fluorescence. We also report the isolation of MUP-IV protein from mouse nasal extracts, in which MUP-IV mRNA has been observed previously. The affinity of each MUP isoform for SBT (K(d) approximately 0.04 to 0.9 micro M) is higher than that for DHB (K(d) approximately 26 to 58 micro M), which in turn is higher than that for HMH (K(d) approximately 50 to 200 micro M). Isoforms I, II, VIII, and IX show very similar affinities for each of the ligands. MUP-VII has approximately twofold higher affinity for SBT but approximately twofold lower affinity for the other pheromones, whereas MUP-IV has approximately 23-fold higher affinity for SBT and approximately fourfold lower affinity for the other pheromones. The variations in ligand affinities of the MUP isoforms are consistent with structural differences in the binding cavities of the isoforms. The data indicate that the concentrations of available pheromones in urine may be influenced by changes in the expression levels of urinary MUPs or the excretion levels of other MUP ligands. The variation in pheromone affinities of the urinary MUP isoforms provides only limited support for the proposal that MUP heterogeneity plays a role in regulating profiles of available pheromones. However, the binding data support the proposed role of nasal MUPs in sequestering pheromones and possibly transporting them to their receptors.
Figures

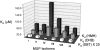
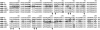
Similar articles
-
High resolution X-ray structures of mouse major urinary protein nasal isoform in complex with pheromones.Protein Sci. 2010 Aug;19(8):1469-79. doi: 10.1002/pro.426. Protein Sci. 2010. PMID: 20509168 Free PMC article.
-
NMR mapping of the recombinant mouse major urinary protein I binding site occupied by the pheromone 2-sec-butyl-4,5-dihydrothiazole.Biochemistry. 1999 Aug 3;38(31):9850-61. doi: 10.1021/bi990497t. Biochemistry. 1999. PMID: 10433691
-
Thermodynamic analysis of binding between mouse major urinary protein-I and the pheromone 2-sec-butyl-4,5-dihydrothiazole.Biochemistry. 2003 May 27;42(20):6302-9. doi: 10.1021/bi026423q. Biochemistry. 2003. PMID: 12755635
-
Comparative study of the molecular variation between 'central' and 'peripheral' MUPs and significance for behavioural signalling.Biochem Soc Trans. 2014 Aug;42(4):866-72. doi: 10.1042/BST20140082. Biochem Soc Trans. 2014. PMID: 25109970 Review.
-
Pheromone signalling in the mouse: role of urinary proteins and vomeronasal organ.Arch Ital Biol. 1999 May;137(2-3):193-200. Arch Ital Biol. 1999. PMID: 10349497 Review.
Cited by
-
The KRAB zinc finger protein RSL1 modulates sex-biased gene expression in liver and adipose tissue to maintain metabolic homeostasis.Mol Cell Biol. 2014 Jan;34(2):221-32. doi: 10.1128/MCB.00875-13. Epub 2013 Nov 4. Mol Cell Biol. 2014. PMID: 24190968 Free PMC article.
-
Spreading of occupational allergens: laboratory animal allergens on hair-covering caps and in mattress dust of laboratory animal workers.Occup Environ Med. 2007 Apr;64(4):267-72. doi: 10.1136/oem.2006.028845. Epub 2006 Oct 19. Occup Environ Med. 2007. PMID: 17053016 Free PMC article.
-
Coordinated expression of the genes Gus and Mup as a potential basis of the functional activity of the androgen-dependent pheromones of the house mouse (Mus musculus L.).Dokl Biol Sci. 2003 Jul-Aug;391:318-21. doi: 10.1023/a:1025194315896. Dokl Biol Sci. 2003. PMID: 14556521 No abstract available.
-
Effects of point mutations in the binding pocket of the mouse major urinary protein MUP20 on ligand affinity and specificity.Sci Rep. 2019 Jan 22;9(1):300. doi: 10.1038/s41598-018-36391-3. Sci Rep. 2019. PMID: 30670733 Free PMC article.
-
Mammalian pheromones.Annu Rev Physiol. 2014;76:151-75. doi: 10.1146/annurev-physiol-021113-170334. Epub 2013 Aug 26. Annu Rev Physiol. 2014. PMID: 23988175 Free PMC article. Review.
References
-
- Al-Shawi, R., Ghazal, P., Clark, A.J., and Bishop, J.O. 1989. Intraspecific evolution of a gene family coding for urinary proteins. J. Mol. Evol. 29 302–313. - PubMed
-
- Bacchini, A., Gaetani, E., and Cavaggioni, A. 1992. Pheromone binding-proteins of the mouse, Mus-musculus. Experientia 48 419–421. - PubMed
-
- Beynon, R., Robertson, D., Hubbard, S.J., Gaskell, S.J., and Hurst, J.L. 1999. The role of protein binding in chemical communication: Major urinary proteins in the house mouse. Advances in chemical communications in vertebrates (ed. R.E. Johnston), pp. 137–147. New York, Plenum Press.
-
- Bocskei, Z., Groon, C.R., Flower, D.R., Wright, C.E., Phillips, S.E.V., Cavaggioni, A., Findlay, J.B.C., and North, A.C.T. 1992. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature 360 186–189. - PubMed
-
- Buck, L. and Axel, R. 1991. A multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65 175–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

