A comparison of transcutaneous bilirubinometers: SpectRx BiliCheck versus Minolta AirShields
- PMID: 12193523
- PMCID: PMC1721445
- DOI: 10.1136/fn.87.2.f137
A comparison of transcutaneous bilirubinometers: SpectRx BiliCheck versus Minolta AirShields
Abstract
Background: Two devices are available for making transcutaneous estimates of serum bilirubin (SBR): the Minolta AirShields JM102 and the new SpectRx BiliCheck.
Objectives: (a) To measure how well the readings produced by these devices agree with SBR measured in the laboratory; (b) to estimate for each device, the proportion of infants with clinical jaundice who would require blood sampling if the device was used as a screening tool to detect infants with SBR > or = 250 micromol/l.
Design: Prospective cohort study of jaundiced infants who required SBR at < or = 20 days of postnatal age. Those who had received phototherapy or exchange transfusion were excluded.
Setting: Tertiary neonatal service in South-East Scotland.
Interventions: Within 30 minutes of SBR sampling, transcutaneous bilirubinometry was performed using one Minolta and two SpectRx devices (designated A and B).
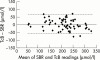
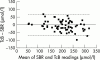
Results: Sixty-four neonates were enrolled, 19 of which were preterm (31-35 weeks). The 95% confidence intervals of a device reading corresponding to SBR were +/- 66.7, +/- 67.9, and +/- 66.4 micromol/l respectively. Using the devices to identify all SBR > or = 250 micromol/l would reduce SBR sampling by 23%, 16%, and 20% respectively.
Conclusions: Given that SBR levels range from 50 to 400 micromol/l in jaundiced infants, the 95% confidence intervals of the devices are wide at +/- 67 micromol/l. The SpectRx can be used as a screening tool for hyperbilirubinaemia but there is no advantage in using it over the Minolta.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
