Isoprenoid biosynthesis in Synechocystis sp. strain PCC6803 is stimulated by compounds of the pentose phosphate cycle but not by pyruvate or deoxyxylulose-5-phosphate
- PMID: 12193620
- PMCID: PMC135332
- DOI: 10.1128/JB.184.18.5045-5051.2002
Isoprenoid biosynthesis in Synechocystis sp. strain PCC6803 is stimulated by compounds of the pentose phosphate cycle but not by pyruvate or deoxyxylulose-5-phosphate
Abstract
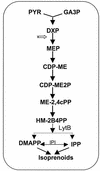
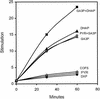
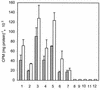
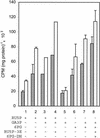
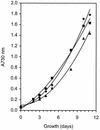
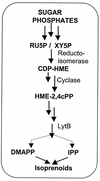
The photosynthetic cyanobacterium Synechocystis sp. strain PCC6803 possesses homologs of known genes of the non-mevalonate 2-C-methyl-D-erythritol 2-phosphate (MEP) pathway for synthesis of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Isoprenoid biosynthesis in extracts of this cyanobacterium, measured by incorporation of radiolabeled IPP, was not stimulated by pyruvate, an initial substrate of the MEP pathway in Escherichia coli, or by deoxyxylulose-5-phosphate, the first pathway intermediate in E. coli. However, high rates of IPP incorporation were obtained with addition of dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GA3P), as well as a variety of pentose phosphate cycle compounds. Fosmidomycin (at 1 micro M and 1 mM), an inhibitor of deoxyxylulose-5-phosphate reductoisomerase, did not significantly inhibit phototrophic growth of the cyanobacterium, nor did it affect [(14)C]IPP incorporation stimulated by DHAP plus GA3P. To date, it has not been possible to unequivocally demonstrate IPP isomerase activity in this cyanobacterium. The combined results suggest that the MEP pathway, as described for E. coli, is not the primary path by which isoprenoids are synthesized under photosynthetic conditions in Synechocystis sp. strain PCC6803. Our data support alternative routes of entry of pentose phosphate cycle substrates derived from photosynthesis.
Figures
References
-
- Altincicek, B., M. Hintz, S. Sanderbrand, J. Wiesner, E. Beck, and H. Jomaa. 2000. Tools for discovery of inhibitors of the 1-deoxy-d-xylulose 5-phosphate (DXP) synthase and DXP reductoisomerase: an approach with enzymes from the pathogenic bacterium Pseudomonas aeruginosa. FEMS Microbiol. Lett. 190:329-333. - PubMed
-
- Altincicek, B., A.-K. Kollas, M. Eberl, J. Wiesner, S. Sanderbrand, M. Hintz, E. Beck, and H. Jomaa. 2001. LytB, a novel gene of the 2-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli. FEBS Lett. 499:37-40. - PubMed
-
- Campos, N., M. Rodriguez-Concepción, M. Seemann, M. Rohmer, and A. Boronat. 2001. Identification of gcpE as a novel gene of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis in Escherichia coli. FEBS Lett. 488:170-173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

