Prospective isolation of human clonogenic common myeloid progenitors
- PMID: 12193648
- PMCID: PMC129361
- DOI: 10.1073/pnas.172384399
Prospective isolation of human clonogenic common myeloid progenitors
Abstract
The hierarchical development from hematopoietic stem cells to mature cells of the hematolymphoid system involves progressive loss of self-renewal capacity, proliferation ability, and lineage potentials. Here we show the prospective isolation of early developmental intermediates, the human clonogenic common myeloid progenitors and their downstream progeny, the granulocyte/macrophage and megakaryocyte/erythrocyte progenitors. All three populations reside in the lineage-negative (lin(-)) CD34(+)CD38(+) fraction of adult bone marrow as well as in cord blood. They are distinguishable by the expression of the IL-3R alpha chain, the receptor of an early-acting hematopoietic cytokine, and CD45RA, an isoform of a phosphotyrosine phosphatase involved in negative regulation of cytokine signaling. Multipotent progenitors, early lymphoid progenitors, and the here-defined myeloid progenitors express distinct profiles of hematopoiesis-affiliated genes. The isolation of highly purified hematopoietic intermediates provides tools to better understand developmental programs underlying normal and leukemic hematopoiesis.
Figures
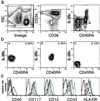



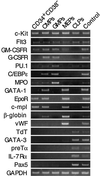
References
-
- Kondo M, Weissman I L, Akashi K. Cell. 1997;91:661–672. - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman I L. Nature (London) 2000;404:193–197. - PubMed
-
- de Wynter E A, Heyworth C M, Mukaida N, Jaworska E, Weffort-Santos A, Matushima K, Testa N G. J Leukocyte Biol. 2001;70:455–460. - PubMed
-
- Debili N, Coulombel L, Croisille L, Katz A, Guichard J, Breton-Gorius J, Vainchenker W. Blood. 1996;88:1284–1296. - PubMed
-
- Debili N, Robin C, Schiavon V, Letestu R, Pflumio F, Mitjavila-Garcia M T, Coulombel L, Vainchenker W. Blood. 2001;97:2023–2030. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

