Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants
- PMID: 12193649
- PMCID: PMC129367
- DOI: 10.1073/pnas.172398899
Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants
Abstract
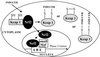
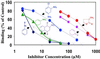
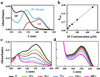
Coordinate induction of phase 2 proteins and elevation of glutathione protect cells against the toxic and carcinogenic effects of electrophiles and oxidants. All inducers react covalently with thiols at rates that are closely related to their potencies. Inducers disrupt the cytoplasmic complex between the actin-bound protein Keap1 and the transcription factor Nrf2, thereby releasing Nrf2 to migrate to the nucleus where it activates the antioxidant response element (ARE) of phase 2 genes and accelerates their transcription. We cloned, overexpressed, and purified murine Keap1 and demonstrated on native gels the formation of complexes of Keap1 with the Neh2 domain of Nrf2 and their concentration-dependent disruption by inducers such as sulforaphane and bis(2-hydroxybenzylidene)acetone. The kinetics, stoichiometry, and order of reactivities of the most reactive of the 25 cysteine thiol groups of Keap1 have been determined by tritium incorporation from [(3)H]dexamethasone mesylate (an inducer and irreversible modifier of thiols) and by UV spectroscopy with sulforaphane, 2,2'-dipyridyl disulfide and 4,4'-dipyridyl disulfide (titrants of thiol groups), and two closely related Michael reaction acceptors [bis(2- and 4-hydroxybenzylidene)acetones] that differ 100-fold in inducer potency and the UV spectra of which are bleached by thiol addition. With large excesses of these reagents nearly all thiols of Keap1 react, but sequential reaction with three successive single equivalents (per cysteine residue) of dipyridyl disulfides revealed excellent agreement with pseudo-first order kinetics, rapid successive declines in reaction velocity, and the stoichiometric formation of two equivalents of thiopyridone per reacted cysteine. This finding suggests that reaction of cysteine thiols is followed by rapid formation of protein disulfide linkages. The most reactive residues of Keap1 (C(257), C(273), C(288), and C(297)) were identified by mapping the dexamethasone-modified cysteines by mass spectrometry of tryptic peptides. These residues are located in the intervening region between BTB and Kelch repeat domains of Keap1 and probably are the direct sensors of inducers of the phase 2 system.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

