Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion
- PMID: 12194856
- PMCID: PMC3369831
- DOI: 10.1016/s1534-5807(02)00216-2
Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion
Abstract
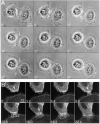
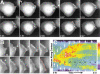
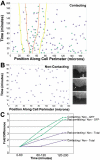
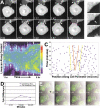
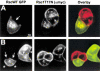
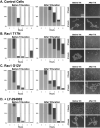
Cadherin-dependent epithelial cell-cell adhesion is thought to be regulated by Rho family small GTPases and PI 3-kinase, but the mechanisms involved are poorly understood. Using time-lapse microscopy and quantitative image analysis, we show that cell-cell contact in MDCK epithelial cells coincides with a spatio-temporal reorganization of plasma membrane Rac1 and lamellipodia from noncontacting to contacting surfaces. Within contacts, Rac1 and lamellipodia transiently concentrate at newest sites, but decrease at older, stabilized sites. Significantly, Rac1 mutants alter kinetics of cell-cell adhesion and strengthening, but not the eventual generation of cell-cell contacts. Products of PI 3-kinase activity also accumulate dynamically at contacts, but are not essential for either initiation or development of cell-cell adhesion. These results define a role for Rac1 in regulating the rates of initiation and strengthening of cell-cell adhesion.
Figures

References
-
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 1998;10:572–577. - PubMed
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 2000;3:445–451. - PubMed
-
- Akhtar N, Hudson KR, Hotchin NA. Co-localization of Rac1 and E-cadherin in human epidermal keratinocytes. Cell Adhes. Commun. 2000;7:465–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

