In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens
- PMID: 12196292
- PMCID: PMC3689299
- DOI: 10.1016/s1074-7613(02)00365-5
In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens
Abstract
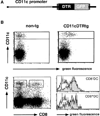
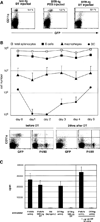
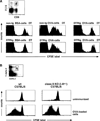
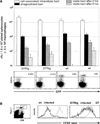
Cytotoxic T lymphocytes (CTL) respond to antigenic peptides presented on MHC class I molecules. On most cells, these peptides are exclusively of endogenous, cytosolic origin. Bone marrow-derived antigen-presenting cells, however, harbor a unique pathway for MHC I presentation of exogenous antigens. This mechanism permits cross-presentation of pathogen-infected cells and the priming of CTL responses against intracellular microbial infections. Here, we report a novel diphtheria toxin-based system that allows the inducible, short-term ablation of dendritic cells (DC) in vivo. We show that in vivo DC are required to cross-prime CTL precursors. Our results thus define a unique in vivo role of DC, i.e., the sensitization of the immune system for cell-associated antigens. DC-depleted mice fail to mount CTL responses to infection with the intracellular bacterium Listeria monocytogenes and the rodent malaria parasite Plasmodium yoelii.
Figures


References
-
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA. Processing of engulfed apoptotic bodies yields T cell epitopes. J. Immunol. 1997;159:5391–5399. - PubMed
-
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. - PubMed
-
- Berg RE, Princiotta MF, Irion S, Moticka JA, Dahl KR, Staerz UD. Positive selection of an H2–M3 restricted T cell receptor. Immunity. 1999;11:33–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

