Proteomic analysis of the mammalian nuclear pore complex
- PMID: 12196509
- PMCID: PMC2173148
- DOI: 10.1083/jcb.200206106
Proteomic analysis of the mammalian nuclear pore complex
Abstract
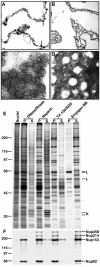
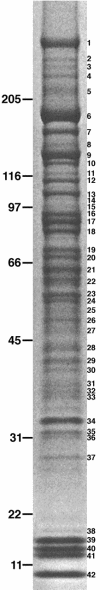
As the sole site of nucleocytoplasmic transport, the nuclear pore complex (NPC) has a vital cellular role. Nonetheless, much remains to be learned about many fundamental aspects of NPC function. To further understand the structure and function of the mammalian NPC, we have completed a proteomic analysis to identify and classify all of its protein components. We used mass spectrometry to identify all proteins present in a biochemically purified NPC fraction. Based on previous characterization, sequence homology, and subcellular localization, 29 of these proteins were classified as nucleoporins, and a further 18 were classified as NPC-associated proteins. Among the 29 nucleoporins were six previously undiscovered nucleoporins and a novel family of WD repeat nucleoporins. One of these WD repeat nucleoporins is ALADIN, the gene mutated in triple-A (or Allgrove) syndrome. Our analysis defines the proteome of the mammalian NPC for the first time and paves the way for a more detailed characterization of NPC structure and function.
Figures

References
-
- Allen, T.D., J.M. Cronshaw, S. Bagley, E. Kiseleva, and M.W. Goldberg. 2000. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell Sci. 113:1651–1659. - PubMed
-
- Blobel, G., and V.R. Potter. 1966. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 154:1662–1665. - PubMed
-
- Cai, S.T., F.L. Zhou, and J.Z. Zhang. 1997. Immunogold labeling electron microscopy showing vimentin filament anchored on nuclear pore complex. Shi Yan Sheng Wu Xue Bao. 30:193–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

