Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels
- PMID: 12196558
- PMCID: PMC6757961
- DOI: 10.1523/JNEUROSCI.22-17-07373.2002
Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels
Abstract
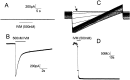
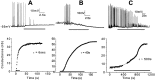
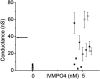
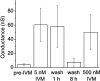
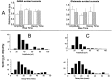
Selectively reducing the excitability of specific neurons will (1) allow for the creation of animal models of human neurological disorders and (2) provide insight into the global function of specific sets of neurons. We focus on a combined genetic and pharmacological approach to silence neurons electrically. We express invertebrate ivermectin (IVM)-sensitive chloride channels (Caenorhabditis elegans GluCl alpha and beta) with a Sindbis virus and then activate these channels with IVM to produce inhibition via a Cl- conductance. We constructed a three-cistron Sindbis virus that expresses the alpha and beta subunits of a glutamate-gated chloride channel (GluCl) along with the green fluorescent protein (EGFP) marker. Expression of the C. elegans channel does not affect the normal spike activity or GABA/glutamate postsynaptic currents of cultured embryonic day 18 hippocampal neurons. At concentrations as low as 5 nm, IVM activates a Cl- current large enough to silence infected neurons effectively. This conductance reverses in 8 hr. These low concentrations of IVM do not potentiate GABA responses. Comparable results are observed with plasmid transfection of yellow fluorescent protein-tagged (EYFP) GluCl alpha and cyan fluorescent protein-tagged (ECFP) GluCl beta. The present study provides an in vitro model mimicking conditions that can be obtained in transgenic mice and in viral-mediated gene therapy. These experiments demonstrate the feasibility of using invertebrate ligand-activated Cl- channels as an approach to modulate excitability.
Figures
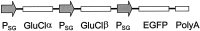


References
-
- Cully DF, Paress PS. Solubilization and characterization of a high-affinity ivermectin binding site from Caenorhabditis elegans. Mol Pharmacol. 1991;40:326–332. - PubMed
-
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
