Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways
- PMID: 12196559
- PMCID: PMC6758007
- DOI: 10.1523/JNEUROSCI.22-17-07380.2002
Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways
Abstract
Tg2576 mice overexpress a mutant form of human amyloid precursor protein with the Swedish mutation (APP(Sw)), resulting in high beta-amyloid (Abeta) levels in the brain. Despite this, amyloid plaques do not develop until 12 months of age, and there is no neuronal loss in mice as old as 16 months. Gene expression profiles in the hippocampus and cerebellum of 6-month-old APP(Sw) mice were compared with age-matched controls. The expression of transthyretin, a protein shown to sequester Abeta and prevent amyloid fibril formation in vitro, and several genes in the insulin-signaling pathway, e.g., insulin-like growth factor-2, were increased selectively in the hippocampus of APP(Sw) mice. Concomitant activation of the insulin-like growth factor-1 receptor, Akt, and extracellular signal-regulated protein kinase 1 and 2 as well as increased phosphorylation of Bad also were unique to the hippocampus of APP(Sw) mice. In addition, the increased expression of transthyretin and insulin-like growth factor-2 and the increased phosphorylation of Bad in hippocampal neurons were maintained in 12-month-old APP(Sw) mice when compared with age-matched controls. These results suggest that the slow progression and lack of full-fledged Alzheimer's disease pathology in the hippocampal neurons of APP(Sw) mice result from the genetic reprogramming of neural cells to cope with increased levels of Abeta.
Figures
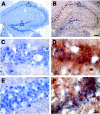
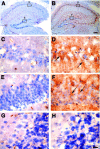
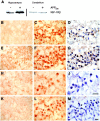
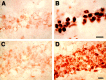


References
-
- Beilharz EJ, Russo VC, Butler G, Baker NL, Connor B, Sirimanne ES, Dragunow M, Werther GA, Gluckman PD, Williams CE, Scheepens A. Co-ordinated and cellular-specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic–ischemic injury. Brain Res Mol Brain Res. 1998;59:119–134. - PubMed
-
- Berlanga JJ, Gualillo O, Buteau H, Applanat M, Kelly PA, Edery M. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J Biol Chem. 1997;272:2050–2052. - PubMed
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. - PubMed
-
- Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. - PubMed
-
- Costoya JA, Finidori J, Moutoussamy S, Senaris R, Devesa J, Arce VM. Activation of growth hormone receptor delivers an antiapoptotic signal: evidence for a role of Akt in this pathway. Endocrinology. 1999;140:5937–5943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
