Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia
- PMID: 12196566
- PMCID: PMC6757997
- DOI: 10.1523/JNEUROSCI.22-17-07444.2002
Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia
Abstract
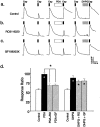
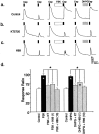
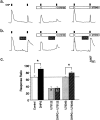
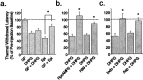
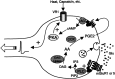
In addition to its role as a CNS neurotransmitter, glutamate has been shown recently to be an important component of the peripheral inflammation response. We demonstrated previously that the group I metabotropic glutamate receptors (mGluRs) mGlu1 and mGlu5 are expressed in the peripheral terminals of sensory neurons and that activation of group I mGluRs in the skin increases thermal sensitivity. In the present study, we provide evidence suggesting that group I mGluRs increase thermal sensitivity by enhancing vanilloid (capsaicin) receptor function. We show that mGlu5 potentiates capsaicin responses in mouse sensory neurons by the phospholipase C pathway but not by activation of protein kinase C. Rather, the effects are mediated by the metabolism of diacylglycerol and the production of prostaglandins via the cyclooxygenase pathway, leading to activation of the cAMP-dependent protein kinase subsequent to prostanoid receptor activation. Behavioral thermal sensitization in mice induced by intraplantar injection of mGlu1/5 agonists was also blocked by inhibitors of protein kinase A and cyclooxygenase, suggesting that a similar signaling pathway operates in vivo. These results demonstrate a novel signaling pathway in sensory neurons and provide a plausible mechanism for the enhancement of thermal sensitivity that occurs with inflammation and after activation of mGluRs on peripheral sensory neuron terminals.
Figures




References
-
- Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. - PubMed
-
- Bley KR, Hunter JC, Eglen RM, Smith JA. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci. 1998;19:141–147. - PubMed
-
- Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. - PubMed
-
- Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
