The myotonia congenita mutation A331T confers a novel hyperpolarization-activated gate to the muscle chloride channel ClC-1
- PMID: 12196568
- PMCID: PMC6758003
- DOI: 10.1523/JNEUROSCI.22-17-07462.2002
The myotonia congenita mutation A331T confers a novel hyperpolarization-activated gate to the muscle chloride channel ClC-1
Abstract
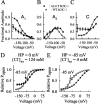
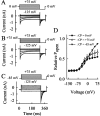
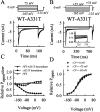
Mutations in the muscle chloride channel gene CLCN1 cause myotonia congenita, an inherited disorder of skeletal muscle excitability leading to a delayed relaxation after muscle contraction. Here, we examine the functional consequences of a novel disease-causing mutation that predicts the substitution of alanine by threonine at position 331 (A331T) by whole-cell patch-clamp recording of recombinant mutant channels. A331T hClC-1 channels exhibit a novel slow gate that activates during membrane hyperpolarization and closes at positive potentials. This novel gate acts in series with fast opening and closing transitions that are common to wild-type (WT) and mutant channels. Under conditions at which this novel gate is not activated, i.e., a holding potential of 0 mV, the typical depolarization-induced activation gating of WT hClC-1 was only slightly affected by the mutation. In contrast, A331T hClC-1 channels with an open slow gate display an altered voltage dependence of open probability. These novel gating features of mutant channels produce a decreased open probability at -85 mV, the normal muscle resting potential, leading to a reduced resting chloride conductance of affected muscle fibers. The A331T mutation causes an unprecedented alteration of ClC-1 gating and reveals novel processes defining transitions between open and closed states in ClC chloride channels.
Figures





References
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. - PubMed
-
- Bryant SH. Muscle membrane of normal and myotonic goats in normal and low external chloride. Fed Proc. 1962;21:312.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
