Targeting axons to specific fiber tracts in vivo by altering cadherin expression
- PMID: 12196585
- PMCID: PMC6758006
- DOI: 10.1523/JNEUROSCI.22-17-07617.2002
Targeting axons to specific fiber tracts in vivo by altering cadherin expression
Abstract
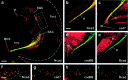
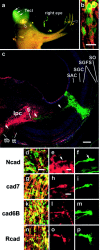
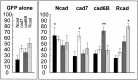
In brain development, neurons have to be connected with specific postsynaptic neurons to establish functional neuronal circuits. Cadherins are cell adhesion molecules, which mark developing neuronal circuits. Each member of this class of molecules is expressed only on a restricted set of fiber fascicles that connect gray matter structures to form functional neural circuits. In view of their expression patterns, cadherins have been postulated to play a functional role in the proper establishment of fiber connections. We chose the chicken optic tectum to analyze the instructive potential of cadherins in axonal pathfinding. Three tectofugal pathways, the tectothalamic, tectobulbar, and tectoisthmic tracts, exit the dorsal mesencephalon via the brachium of the superior colliculus, a large fiber structure, which can be divided in specific subtracts that are characterized by the selective expression of N-cadherin, cadherin-7, cadherin-6B, or R-cadherin. By using in vivo electroporation, we overexpressed each of the cadherins in tectal projection neurons between embryonic days 6 and 11. Cotransfection with green fluorescent protein expression plasmid allowed us to assess the pathway choice, which the transgenic axons had made. Quantification based on confocal laser scanning microscopic images revealed that transgenic axons selectively fasciculated with tectofugal tracts specified by the matching type of cadherin. This is the first direct evidence that cadherins mediate differential axonal pathfinding in vivo, possibly by a preferentially homotypic adhesive mechanism.
Figures



Similar articles
-
Expression of R-cadherin and N-cadherin by cell groups and fiber tracts in the developing mouse forebrain: relation to the formation of functional circuits.Neuroscience. 2001;106(3):505-33. doi: 10.1016/s0306-4522(01)00292-5. Neuroscience. 2001. PMID: 11591453
-
Combinatorial expression of cadherins in the tectum and the sorting of neurites in the tectofugal pathways of the chicken embryo.Neuroscience. 1999 Mar;90(3):985-1000. doi: 10.1016/s0306-4522(98)00526-0. Neuroscience. 1999. PMID: 10218798
-
Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain.J Comp Neurol. 1993 Jul 15;333(3):398-416. doi: 10.1002/cne.903330307. J Comp Neurol. 1993. PMID: 7688773
-
Cadherins in the central nervous system.Prog Neurobiol. 2000 Aug;61(6):611-48. doi: 10.1016/s0301-0082(99)00070-2. Prog Neurobiol. 2000. PMID: 10775799 Review.
-
Cadherin expression in the developing vertebrate CNS: from neuromeres to brain nuclei and neural circuits.Exp Cell Res. 1995 Oct;220(2):243-56. doi: 10.1006/excr.1995.1313. Exp Cell Res. 1995. PMID: 7556431 Review.
Cited by
-
Enhanced cell-cell contact stability and decreased N-cadherin-mediated migration upon fibroblast growth factor receptor-N-cadherin cross talk.Oncogene. 2019 Aug;38(35):6283-6300. doi: 10.1038/s41388-019-0875-6. Epub 2019 Jul 16. Oncogene. 2019. PMID: 31312021
-
Restricted expression of classic cadherins in the spinal cord of the chicken embryo.Front Neuroanat. 2014 Mar 31;8:18. doi: 10.3389/fnana.2014.00018. eCollection 2014. Front Neuroanat. 2014. PMID: 24744704 Free PMC article.
-
Loss of retinal cadherin facilitates mammary tumor progression and metastasis.Cancer Res. 2009 Jun 15;69(12):5030-8. doi: 10.1158/0008-5472.CAN-08-4007. Epub 2009 Jun 2. Cancer Res. 2009. PMID: 19491271 Free PMC article.
-
R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons.J Neurosci. 2003 Oct 29;23(30):9873-80. doi: 10.1523/JNEUROSCI.23-30-09873.2003. J Neurosci. 2003. PMID: 14586016 Free PMC article.
-
Distinct PTPmu-associated signaling molecules differentially regulate neurite outgrowth on E-, N-, and R-cadherin.Mol Cell Neurosci. 2010 May;44(1):78-93. doi: 10.1016/j.mcn.2010.02.005. Epub 2010 Mar 1. Mol Cell Neurosci. 2010. PMID: 20197094 Free PMC article.
References
-
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. - PubMed
-
- Broadbent ID, Pettitt J. The C. elegans hmr-1 gene can encode a neuronal classic cadherin involved in the regulation of axon fasciculation. Curr Biol. 2002;12:59–63. - PubMed
-
- Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
