Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo
- PMID: 12196587
- PMCID: PMC6757994
- DOI: 10.1523/JNEUROSCI.22-17-07639.2002
Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo
Abstract
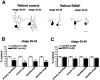
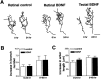
The dendritic and axonal arbors of developing retinal ganglion cells (RGCs) are exposed to two sources of BDNF: RGC dendrites are exposed to BDNF locally within the retina, and RGC axons are exposed to BDNF at the target, the optic tectum. Our previous studies demonstrated that increasing tectal BDNF levels promotes RGC axon terminal arborization, whereas increasing retinal BDNF levels inhibits RGC dendritic arborization. These results suggested that differential neurotrophic action at the axon versus dendrite might be responsible for the opposing effects of BDNF on RGC axonal versus dendritic arborization. To explore this possibility, we examined the effects of altering BDNF levels at the optic tectum on the elaboration of RGC dendritic arbors in the retina. Increasing tectal BDNF levels resulted in a significant increase in dendritic branching, whereas neutralizing endogenous tectal BDNF with function-blocking antibodies significantly decreased dendritic arbor complexity. Thus, RGC dendritic arbors react in opposing manners to retinal- versus tectal-derived BDNF. Alterations in retinal BDNF levels, however, did not affect axon terminal arborization. Thus, RGC dendritic arborization is controlled in a complementary manner by both local and target-derived sources of BDNF, whereas axon arborization is modulated solely by neurotrophic interactions at the target. Together, our results indicate that developing RGCs modulate dendritic arborization by integrating signals from discrete sources of BDNF in the eye and brain. Differential integration of spatially discrete neurotrophin signals within a single neuron may therefore finely tune afferent and efferent neuronal connectivity.
Figures
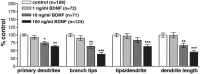
Similar articles
-
Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo.J Neurosci. 1999 Nov 15;19(22):9928-38. doi: 10.1523/JNEUROSCI.19-22-09928.1999. J Neurosci. 1999. PMID: 10559401 Free PMC article.
-
BDNF increases synapse density in dendrites of developing tectal neurons in vivo.Development. 2006 Jul;133(13):2477-86. doi: 10.1242/dev.02409. Epub 2006 May 25. Development. 2006. PMID: 16728478
-
Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity.J Neurosci. 2007 Mar 7;27(10):2444-56. doi: 10.1523/JNEUROSCI.4434-06.2007. J Neurosci. 2007. PMID: 17344382 Free PMC article.
-
BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death.J Neurosci. 1998 Mar 15;18(6):2097-107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. J Neurosci. 1998. PMID: 9482796 Free PMC article. Review.
-
Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses.Int J Dev Biol. 2004;48(8-9):947-56. doi: 10.1387/ijdb.041883sc. Int J Dev Biol. 2004. PMID: 15558485 Review.
Cited by
-
Voluntary exercise increases axonal regeneration from sensory neurons.Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8473-8. doi: 10.1073/pnas.0401443101. Epub 2004 May 24. Proc Natl Acad Sci U S A. 2004. PMID: 15159540 Free PMC article.
-
Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats.J Neurosci. 2003 Aug 6;23(18):7034-44. doi: 10.1523/JNEUROSCI.23-18-07034.2003. J Neurosci. 2003. PMID: 12904464 Free PMC article.
-
Automated Sholl analysis of digitized neuronal morphology at multiple scales: Whole cell Sholl analysis versus Sholl analysis of arbor subregions.Cytometry A. 2010 Dec;77(12):1160-8. doi: 10.1002/cyto.a.20954. Cytometry A. 2010. PMID: 20687200 Free PMC article.
-
Glial cells as a promising therapeutic target of glaucoma: beyond the IOP.Front Ophthalmol (Lausanne). 2024 Jan 8;3:1310226. doi: 10.3389/fopht.2023.1310226. eCollection 2023. Front Ophthalmol (Lausanne). 2024. PMID: 38983026 Free PMC article. Review.
-
Spike-timing-dependent BDNF secretion and synaptic plasticity.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130132. doi: 10.1098/rstb.2013.0132. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298135 Free PMC article.
References
-
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. - PubMed
-
- Bahr M. Live or let die: retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. - PubMed
-
- Bahr M, Wizenmann A, Thanos S. Effect of bilateral tectum lesions on retinal ganglion cell morphology in rats. J Comp Neurol. 1992;320:370–380. - PubMed
-
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite growth by nerve growth factor. Dev Biol. 1982;93:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
