Untranslated element in neurofilament mRNA has neuropathic effect on motor neurons of transgenic mice
- PMID: 12196589
- PMCID: PMC6758005
- DOI: 10.1523/JNEUROSCI.22-17-07662.2002
Untranslated element in neurofilament mRNA has neuropathic effect on motor neurons of transgenic mice
Abstract
Studies of experimental motor neuron degeneration attributable to expression of neurofilament light chain (NF-L) transgenes have raised the possibility that the neuropathic effects result from overexpression of NF-L mRNA, independent of NF-L protein effects (Cañete-Soler et al., 1999). The present study was undertaken to test for an RNA-mediated pathogenesis. Transgenic mice were derived using either an enhanced green fluorescent protein reporter construct or modified chimeric constructs that differ only in their 3' untranslated regions (UTRs). Motor function and spinal cord histology were normal in mice expressing the unmodified reporter transgene. In mice expressing a chimeric transgene in which sequence of NF-L 3' UTR was inserted into the 3' UTR of the reporter transgene, we observed growth retardation and reduced kinetic activity during postnatal development. Older mice developed impairment of motor function and atrophy of nerve fibers in the ventral roots. A similar but more severe phenotype was observed when the chimeric transgene contained a 36 bp c-myc insert in an mRNA destabilizing element of the NF-L sequence. Our results suggest that neuropathic effects of overexpressing NF-L can occur at the level of transgene RNA and are mediated by sequences in the NF-L 3' UTR.
Figures



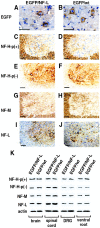

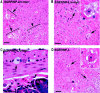
Similar articles
-
Mutation in neurofilament transgene implicates RNA processing in the pathogenesis of neurodegenerative disease.J Neurosci. 1999 Feb 15;19(4):1273-83. doi: 10.1523/JNEUROSCI.19-04-01273.1999. J Neurosci. 1999. PMID: 9952405 Free PMC article.
-
RNA-binding protein is involved in aggregation of light neurofilament protein and is implicated in the pathogenesis of motor neuron degeneration.Hum Mol Genet. 2005 Dec 1;14(23):3643-59. doi: 10.1093/hmg/ddi392. Epub 2005 Oct 19. Hum Mol Genet. 2005. PMID: 16236762
-
Similar poly(C)-sensitive RNA-binding complexes regulate the stability of the heavy and light neurofilament mRNAs.Brain Res. 2000 Jun 9;867(1-2):265-79. doi: 10.1016/s0006-8993(00)02389-1. Brain Res. 2000. PMID: 10837825
-
Post-transcriptional control of neurofilaments in development and disease.Exp Cell Res. 2007 Jun 10;313(10):2088-97. doi: 10.1016/j.yexcr.2007.02.014. Epub 2007 Feb 27. Exp Cell Res. 2007. PMID: 17428473 Review.
-
Cytoskeletal abnormalities in amyotrophic lateral sclerosis: beneficial or detrimental effects?J Neurol Sci. 2000 Nov 1;180(1-2):7-14. doi: 10.1016/s0022-510x(00)00422-6. J Neurol Sci. 2000. PMID: 11090858 Review.
Cited by
-
Dysfunctions of neuronal and glial intermediate filaments in disease.J Clin Invest. 2009 Jul;119(7):1814-24. doi: 10.1172/JCI38003. Epub 2009 Jul 1. J Clin Invest. 2009. PMID: 19587456 Free PMC article. Review.
-
The emerging role of guanine nucleotide exchange factors in ALS and other neurodegenerative diseases.Front Cell Neurosci. 2014 Sep 10;8:282. doi: 10.3389/fncel.2014.00282. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25309324 Free PMC article. Review.
-
Posttranscriptional regulation of neurofilament proteins and tau in health and disease.Brain Res Bull. 2023 Jan;192:115-127. doi: 10.1016/j.brainresbull.2022.10.017. Epub 2022 Oct 29. Brain Res Bull. 2023. PMID: 36441047 Free PMC article. Review.
-
The role of RNA and RNA processing in neurodegeneration.J Neurosci. 2005 Nov 9;25(45):10372-5. doi: 10.1523/JNEUROSCI.3453-05.2005. J Neurosci. 2005. PMID: 16280575 Free PMC article. Review. No abstract available.
-
Aldolases a and C are ribonucleolytic components of a neuronal complex that regulates the stability of the light-neurofilament mRNA.J Neurosci. 2005 Apr 27;25(17):4353-64. doi: 10.1523/JNEUROSCI.0885-05.2005. J Neurosci. 2005. PMID: 15858061 Free PMC article.
References
-
- Abel A, Walcott J, Woods J, Duda J, Merry DE. Expression of expanded repeat androgen receptor produces neurologic disease in transgenic mice. Hum Mol Genet. 2001;10:107–110. - PubMed
-
- Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Do J, Sang C, Kobayashi Y, Doyu M, Sobue G. Transgenic mice with an expanded CAG repeat controlled by the human AR promoter show polyglutamine nuclear inclusions and neuronal dysfunction without neuronal cell death. Hum Mol Genet. 2001;10:1039–1048. - PubMed
-
- Akamatsu W, Okano HJ, Osumi N, Inoue T, Nakamura S, Sakakibara S, Miura M, Matsuo N, Darnell RB, Okano H. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HUC promote neuronal development in both the central and the peripheral nervous systems. Proc Natl Acad Sci USA. 1999;17:9885–9890. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
