Adenosine induces inositol 1,4,5-trisphosphate receptor-mediated mobilization of intracellular calcium stores in basal forebrain cholinergic neurons
- PMID: 12196591
- PMCID: PMC6758010
- DOI: 10.1523/JNEUROSCI.22-17-07680.2002
Adenosine induces inositol 1,4,5-trisphosphate receptor-mediated mobilization of intracellular calcium stores in basal forebrain cholinergic neurons
Abstract
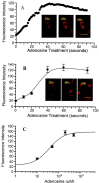
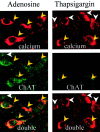
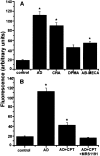
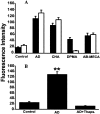
In the cholinergic basal forebrain, we found previously that the extracellular adenosine concentration increase that accompanies sleep deprivation, acting via the A1 receptor, led to activation of the transcription factor nuclear factor-kappaB and to the upregulation of A1 adenosine receptor mRNA. We thus began to examine intracellular signaling mechanisms. We report here that adenosine, acting in a dose-dependent manner and predominantly via A1 receptors, stimulated IP3 receptor-regulated calcium release from intracellular stores. To the best of our knowledge, this calcium signaling pathway effect is a novel action of the G(i)-coupled A1 adenosine receptor in neurons. Moreover, this calcium mobilization was not seen at all in noncholinergic neurons but was present in a large proportion of cholinergic neurons. These data suggest a potential role for a calcium-signaling pathway in adenosine-induced long-term effects of sleep deprivation and a key role for cholinergic neurons in this process.
Figures
References
-
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, Cattabeni F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. - PubMed
-
- Bading H. Transcription-dependent neuronal plasticity: the nuclear calcium hypothesis. Eur J Biochem. 2000;267:5280–5283. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain Res Mol Brain Res. 1999;73:1–10. - PubMed
-
- Basheer R, Porkka-Heiskanen T, Strecker RE, Thakkar MM, McCarley RW. Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biol Signals Recept. 2000;9:319–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
