Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control
- PMID: 12196593
- PMCID: PMC6757981
- DOI: 10.1523/JNEUROSCI.22-17-07695.2002
Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control
Abstract
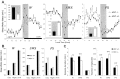
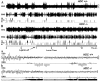
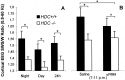
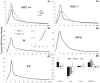
The hypothesis that histaminergic neurons are involved in brain arousal is supported by many studies. However, the effects of the selective long-term abolition of histaminergic neurons on the sleep-wake cycle, indispensable in determining their functions, remain unknown. We have compared brain histamine(HA)-immunoreactivity and the cortical-EEG and sleep-wake cycle under baseline conditions or after behavioral or pharmacological stimuli in wild-type (WT) and knock-out mice lacking the histidine decarboxylase gene (HDC-/-). HDC-/-mice showed an increase in paradoxical sleep, a decrease in cortical EEG power in theta-rhythm during waking (W), and a decreased EEG slow wave sleep/W power ratio. Although no major difference was noted in the daily amount of spontaneous W, HDC-/-mice showed a deficit of W at lights-off and signs of somnolence, as demonstrated by a decreased sleep latencies after various behavioral stimuli, e.g., WT-mice placed in a new environment remained highly awake for 2-3 hr, whereas HDC-/-mice fell asleep after a few minutes. These effects are likely to be attributable to lack of HDC and thus of HA. In WT mice, indeed, intraperitoneal injection of alpha-fluoromethylhistidine (HDC-inhibitor) caused a decrease in W, whereas injection of ciproxifan (HA-H3 receptor antagonist) elicited W. Both injections had no effect in HDC-/-mice. Moreover, PCR and immunohistochemistry confirmed the absence of the HDC gene and brain HA-immunoreactive neurons in the HDC-/-mice. These data indicate that disruption of HA-synthesis causes permanent changes in the cortical-EEG and sleep-wake cycle and that, at moments when high vigilance is required (lights off, environmental change em leader ), mice lacking brain HA are unable to remain awake, a prerequisite condition for responding to behavioral and cognitive challenges. We suggest that histaminergic neurons also play a key role in maintaining the brain in an awake state faced with behavioral challenges.
Figures







Similar articles
-
Role of histamine H1-receptor on behavioral states and wake maintenance during deficiency of a brain activating system: A study using a knockout mouse model.Neuropharmacology. 2016 Jul;106:20-34. doi: 10.1016/j.neuropharm.2015.12.014. Epub 2015 Dec 23. Neuropharmacology. 2016. PMID: 26723880
-
Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models.J Neurosci. 2009 Nov 18;29(46):14423-38. doi: 10.1523/JNEUROSCI.2604-09.2009. J Neurosci. 2009. PMID: 19923277 Free PMC article.
-
Enhanced histaminergic neurotransmission and sleep-wake alterations, a study in histamine H3-receptor knock-out mice.Neuropsychopharmacology. 2013 May;38(6):1015-31. doi: 10.1038/npp.2012.266. Epub 2013 Jan 2. Neuropsychopharmacology. 2013. PMID: 23303066 Free PMC article.
-
Modulation of behavior by the histaminergic system: lessons from HDC-, H3R- and H4R-deficient mice.Neurosci Biobehav Rev. 2014 Nov;47:101-21. doi: 10.1016/j.neubiorev.2014.07.020. Epub 2014 Aug 4. Neurosci Biobehav Rev. 2014. PMID: 25102165 Review.
-
[From biochemistry to pharmacology: the histaminergic neuron system in the brain].Nihon Yakurigaku Zasshi. 1992 Feb;99(2):63-81. doi: 10.1254/fpj.99.63. Nihon Yakurigaku Zasshi. 1992. PMID: 1559640 Review. Japanese.
Cited by
-
Levels of Interference in Long and Short-Term Memory Differentially Modulate Non-REM and REM Sleep.Sleep. 2016 Dec 1;39(12):2173-2188. doi: 10.5665/sleep.6322. Sleep. 2016. PMID: 27748246 Free PMC article.
-
Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand.J Neurosci. 2011 Dec 14;31(50):18439-52. doi: 10.1523/JNEUROSCI.2676-11.2011. J Neurosci. 2011. PMID: 22171046 Free PMC article.
-
Body Weight and Metabolic Rate Changes in Narcolepsy: Current Knowledge and Future Directions.Metabolites. 2022 Nov 16;12(11):1120. doi: 10.3390/metabo12111120. Metabolites. 2022. PMID: 36422261 Free PMC article. Review.
-
Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice.J Neurosci. 2010 Mar 24;30(12):4382-9. doi: 10.1523/JNEUROSCI.4936-09.2010. J Neurosci. 2010. PMID: 20335474 Free PMC article.
-
Regional expression of P2Y(4) receptors in the rat central nervous system.Purinergic Signal. 2011 Dec;7(4):469-88. doi: 10.1007/s11302-011-9246-7. Epub 2011 Jul 19. Purinergic Signal. 2011. PMID: 21769584 Free PMC article.
References
-
- Airaksinen MS, Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988;273:163–186. - PubMed
-
- Airaksinen MS, Alanen S, Szabat E, Visser TJ, Panula P. Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse, and guinea pig. J Comp Neurol. 1992;323:103–116. - PubMed
-
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. - PubMed
-
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Saunders; Philadelphia: 2000. pp. 377–390.
-
- Bouthenet ML, Ruat M, Sales N, Garbarg M, Schwartz JC. A detailed mapping of histamine H1-receptors in guinea-pig central nervous system established by autoradiography with [125I]iodobolpyramine. Neuroscience. 1988;26:553–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
