Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization
- PMID: 12196597
- PMCID: PMC6757977
- DOI: 10.1523/JNEUROSCI.22-17-07737.2002
Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization
Abstract
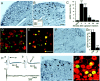
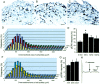
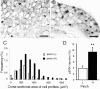
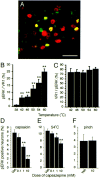
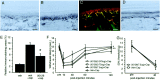
Alteration in the intracellular signal transduction pathway in primary afferent neurons may contribute to pain hypersensitivity. We demonstrated that very rapid phosphorylation of extracellular signal-regulated protein kinases (pERK) occurred in DRG neurons that were taking part in the transmission of various noxious signals. The electrical stimulation of Adelta fibers induced pERK primarily in neurons with myelinated fibers. c-Fiber activation by capsaicin injection induced pERK in small neurons with unmyelinated fibers containing vanilloid receptor-1 (VR-1), suggesting that pERK labeling in DRG neurons is modality specific. Electrical stimulation at the c-fiber level with different intensities and frequencies revealed that phosphorylation of ERK is dependent on the frequency. We examined the pERK in the DRG after application of natural noxious stimuli and found a stimulus intensity-dependent increase in labeled cell size and in the number of activated neurons in the c- and Adelta-fiber population. Immunohistochemical double labeling with phosphorylated ERK/VR-1 and pharmacological study demonstrated that noxious heat stimulation induced pERK in primary afferents in a VR-1-dependent manner. Capsaicin injection into the skin also increased pERK labeling significantly in peripheral fibers and terminals in the skin, which was prevented by a mitogen-activated protein kinase/ERK kinase inhibitor, 1,4-diamino-2,3-dicyano-1,4-bis(2-aminopheylthio)butadiene (U0126). Behavioral experiments showed that U0126 dose-dependently attenuated thermal hyperalgesia after capsaicin injection and suggested that the activation of ERK pathways in primary afferent neurons is involved in the sensitization of primary afferent neurons. Thus, pERK in primary afferents by noxious stimulation in vivo showed distinct characteristics of expression and may be correlated with the functional activity of primary afferent neurons.
Figures

References
-
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
-
- Averill S, Delcroix JD, Michael GJ, Tomlinson DR, Fernyhough P, Priestley JV. Nerve growth factor modulates the activation status and fast axonal transport of ERK 1/2 in adult nociceptive neurons. Mol Cell Neurosci. 2001;18:183–196. - PubMed
-
- Baraban J, Fiore RS, Sanghera JS, Paddon HB, Pelech SL. Identification of p42 mitogen-activated protein kinase as a tyrosine kinase substrate activated by maximal electroconvulsive shock in hippocampus. J Neurochem. 1993;60:330–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
