The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG
- PMID: 12196628
- PMCID: PMC129402
- DOI: 10.1073/pnas.142433199
The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG
Abstract
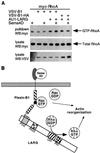
Semaphorins are axon guidance molecules that signal through the plexin family of receptors. Semaphorins also play a role in other processes such as immune regulation and tumorigenesis. However, the molecular signaling mechanisms downstream of plexin receptors have not been elucidated. Semaphorin 4D is the ligand for the plexin-B1 receptor and stimulation of the plexin-B1 receptor activates the small GTPase RhoA. Using the intracellular domain of plexin-B1 as an affinity ligand, two Rho-specific guanine nucleotide exchange factors, leukemia-associated Rho GEF (LARG; GEF, guanine nucleotide exchange factors) and PSD-95/Dlg/ZO-1 homology (PDZ)-RhoGEF, were isolated from mouse brain as plexin-B1-specific interacting proteins. LARG and PDZ-RhoGEF contain several functional domains, including a PDZ domain. Biochemical characterizations showed that the PDZ domain of LARG is directly involved in the interaction with the carboxy-terminal sequence of plexin-B1. Mutation of either the PDZ domain in LARG or the PDZ binding site in plexin-B1 eliminates the interaction. The interaction between plexin-B1 and LARG is specific for the PDZ domain of LARG and LARG does not interact with plexin-A1. A LARG-interaction defective mutant of the plexin-B1 receptor was created and was unable to stimulate RhoA activation. The data in this report suggest that LARG plays a critical role in plexin-B1 signaling to stimulate Rho activation and cytoskeletal reorganization.
Figures


References
-
- Luo Y., Raible, D. & Raper, J. A. (1993) Cell 75, 217-227. - PubMed
-
- Kolodkin A. L., Levengood, D. V., Rowe, E. G., Tai, Y. T., Giger, R. J. & Ginty, D. D. (1997) Cell 90, 753-762. - PubMed
-
- Christensen C. R., Klingelhofer, J., Tarabykina, S., Hulgaard, E. F., Kramerov, D. & Lukanidin, E. (1998) Cancer Res. 58, 1238-1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

