Neocentromeres: role in human disease, evolution, and centromere study
- PMID: 12196915
- PMCID: PMC378529
- DOI: 10.1086/342730
Neocentromeres: role in human disease, evolution, and centromere study
Abstract
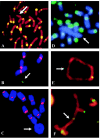
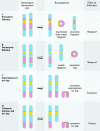
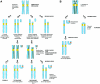
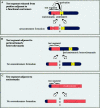
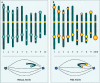
The centromere is essential for the proper segregation and inheritance of genetic information. Neocentromeres are ectopic centromeres that originate occasionally from noncentromeric regions of chromosomes. Despite the complete absence of normal centromeric alpha-satellite DNA, human neocentromeres are able to form a primary constriction and assemble a functional kinetochore. Since the discovery and characterization of the first case of a human neocentromere in our laboratory a decade ago, 60 examples of constitutional human neocentromeres distributed widely across the genome have been described. Typically, these are located on marker chromosomes that have been detected in children with developmental delay or congenital abnormalities. Neocentromeres have also been detected in at least two types of human cancer and have been experimentally induced in Drosophila. Current evidence from human and fly studies indicates that neocentromere activity is acquired epigenetically rather than by any alteration to the DNA sequence. Since human neocentromere formation is generally detrimental to the individual, its biological value must lie beyond the individual level, such as in karyotype evolution and speciation.
Figures

References
-
- Aagaard L, Schmid M, Warburton P, Jenuwein T (2000) Mitotic phosphorylation of SUV39H1, a novel component of active centromeres, coincides with transient accumulation at mammalian centromeres. J Cell Sci 113:817–829 - PubMed
-
- Abeliovich D, Yehuda O, Ben-Neriah S, Kapelushnik Y, Ben-Yehuda D (1996) dup(10q) lacking alpha-satellite DNA in bone marrow cells of a patient with acute myeloid leukemia. Cancer Genet Cytogenet 89:1–6 - PubMed
-
- Assumpção JG, Berkofsky-Fessler W, Campos NV, Macial-Guerra AT, Li S, Melaragno MI, de Mello MP, Warburton PE. Identification of a neocentromere in a rearranged Y chromosome with no detectable DY23 centromeric sequence. Am J Med Genet (in press) - PubMed
-
- Barbi G, Kennerknecht I, Wohr G, Avramopoulos D, Karadima G, Petersen MB (2000) Mirror-symmetric duplicated chromosome 21q with minor proximal deletion, and with neocentromere in a child without the classical Down syndrome phenotype. Am J Med Genet 91:116–122 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

