Biophysical limitation of cell elongation in cereal leaves
- PMID: 12197513
- PMCID: PMC4240423
- DOI: 10.1093/aob/mcf180
Biophysical limitation of cell elongation in cereal leaves
Abstract
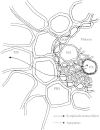
Grass leaves grow from the base. Unlike those of dicotyledonous plants, cells of grass leaves expand enclosed by sheaths of older leaves, where there is little or no transpiration, and go through developmental stages in a strictly linear arrangement. The environmental or developmental factor that limits leaf cell expansion must do so through biophysical means at the cellular level: wall-yielding, water uptake and solute supply are all candidates. This Botanical Briefing looks at the possibility that tissue hydraulic conductance limits cell expansion and leaf growth. A model is presented that relates pathways of water movement in the elongation zone of grass leaves to driving forces for water movement and to anatomical features. The bundle sheath is considered as a crucial control point. The relative importance of these pathways for the regulation of leaf growth and for the partitioning of water between expansion and transpiration is discussed.
Figures

Similar articles
-
Biophysical limitation of leaf cell elongation in source-reduced barley.Planta. 2002 Jun;215(2):327-38. doi: 10.1007/s00425-002-0747-z. Epub 2002 Mar 20. Planta. 2002. PMID: 12029483
-
Limitation of Cell Elongation in Barley (Hordeum vulgare L.) Leaves Through Mechanical and Tissue-Hydraulic Properties.Plant Cell Physiol. 2015 Jul;56(7):1364-73. doi: 10.1093/pcp/pcv055. Epub 2015 Apr 22. Plant Cell Physiol. 2015. PMID: 25907571
-
The biophysics of leaf growth in salt-stressed barley. A study at the cell level.Plant Physiol. 2002 May;129(1):374-88. doi: 10.1104/pp.001164. Plant Physiol. 2002. PMID: 12011367 Free PMC article.
-
The short-term growth response to salt of the developing barley leaf.J Exp Bot. 2006;57(5):1079-95. doi: 10.1093/jxb/erj095. Epub 2006 Mar 2. J Exp Bot. 2006. PMID: 16513814 Review.
-
Wall relaxation and the driving forces for cell expansive growth.Plant Physiol. 1987;84(3):561-4. doi: 10.1104/pp.84.3.561. Plant Physiol. 1987. PMID: 11539680 Free PMC article. Review.
Cited by
-
Nitrogen recycling from the xylem in rice leaves: dependence upon metabolism and associated changes in xylem hydraulics.J Exp Bot. 2016 Apr;67(9):2901-11. doi: 10.1093/jxb/erw132. Epub 2016 Apr 6. J Exp Bot. 2016. PMID: 27053722 Free PMC article.
-
Cuticular wax deposition in growing barley (Hordeum vulgare) leaves commences in relation to the point of emergence of epidermal cells from the sheaths of older leaves.Planta. 2005 Oct;222(3):472-83. doi: 10.1007/s00425-005-1552-2. Epub 2005 Jun 7. Planta. 2005. PMID: 15940461
-
Regulation of leaf hydraulics: from molecular to whole plant levels.Front Plant Sci. 2013 Jul 15;4:255. doi: 10.3389/fpls.2013.00255. eCollection 2013. Front Plant Sci. 2013. PMID: 23874349 Free PMC article.
-
Effects of the semi-dwarfing sdw1/denso gene in barley.J Appl Genet. 2013 Nov;54(4):381-90. doi: 10.1007/s13353-013-0165-x. Epub 2013 Aug 22. J Appl Genet. 2013. PMID: 23975516 Free PMC article. Review.
-
Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture.Plant Physiol. 2011 Jun;156(2):832-43. doi: 10.1104/pp.111.173856. Epub 2011 Apr 21. Plant Physiol. 2011. PMID: 21511989 Free PMC article.
References
-
- ArifH, Tomos AD.1993. Control of wheat leaf growth under saline conditions. In: Lieth H, Al Masoom H, eds. Towards the rational use of high salinity tolerant plants. Vol 2 Dordrecht, The Netherlands: Kluwer Academic Publishers, 45–52.
-
- BarlowEWR.1986. Water relations of expanding leaves. Australian Journal of Plant Physiology 13: 45–58.

