Treatment of oral pemphigoid with intravenous immunoglobulin as monotherapy. Long-term follow-up: influence of treatment on antibody titres to human alpha6 integrin
- PMID: 12197896
- PMCID: PMC1906475
- DOI: 10.1046/j.1365-2249.2002.01942.x
Treatment of oral pemphigoid with intravenous immunoglobulin as monotherapy. Long-term follow-up: influence of treatment on antibody titres to human alpha6 integrin
Abstract
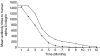
Oral pemphigoid (OP) is a chronic autoimmune disease, involving the oral cavity, characterized by a homogenous linear deposition of immunoglobulins, complement, or both along the basement membrane zone (BMZ) and a subepithelial blister formation. The alpha6/beta4 heterodimer is an integrin family of adhesion receptors, which mediates basal cell to matrix interactions. Recent evidence suggests a pathophysiologic role for antibodies against human alpha6 integrin in blister formation in OP, in organ culture studies. Fifty percent of OP patients have been reported to experience disease progression to involve other mucosal tissues, including the eye and larynx. To prevent this extension of disease, systemic therapy with systemic corticosteroids, dapsone, and immunosuppressive agents has been recommended. The use of intravenous immunoglobulin (IVIg) in the treatment of pemphigoid has been recently described. In this study, we present the use of IVIg, in a group of seven patients, with severe OP, in whom systemic conventional treatment was contraindicated. To determine the influence of treatment on antibodies to human alpha6 integrin in OP, seven patients with OP treated with IVIg therapy and a comparable control group of seven patients with OP, treated with conventional therapy, were evaluated at monthly intervals, for a 12 consecutive month treatment period. An effective clinical response was observed in all seven patients treated with IVIg therapy, after a mean treatment period of 4.5 months. IVIg therapy induced a prolonged and sustained clinical remission in all seven patients after a mean treatment period of 26.9 months. A statistically significant difference was observed in the quality of life pre- and post-IVIg therapy (P < 0.001). Both the study and the control groups had a very similar initial serological response to treatment. A statistically significant reduction in the antibody titres was observed after four months of treatment, in both groups (P = 0.015). Thereafter, patients treated with IVIg therapy had a faster rate of decline in the antibody titres, and the difference in the rate of decline between the study and control groups became statistically significant after six months of treatment (P = 0.03). The use of IVIg therapy resulted in reduction of antialpha6 antibody titres and in inducing and maintaining both a sustained, clinical and serological remission.
Figures


Comment in
-
High-dose intravenous immunoglobulin (hdIVIg) in the treatment of autoimmune blistering disorders.Clin Exp Immunol. 2002 Sep;129(3):385-9. doi: 10.1046/j.1365-2249.2002.01967.x. Clin Exp Immunol. 2002. PMID: 12197877 Free PMC article. No abstract available.
Similar articles
-
Influence of intravenous immunoglobulin therapy on autoantibody titres to BP Ag1 and BP Ag2 in patients with bullous pemphigoid.J Eur Acad Dermatol Venereol. 2003 Nov;17(6):641-5. doi: 10.1046/j.1468-3083.2003.00714.x. J Eur Acad Dermatol Venereol. 2003. PMID: 14761129
-
Comparison of reactivity and epitope recognition between sera from American and Italian patients with oral pemphigoid.Clin Exp Immunol. 2006 Jul;145(1):28-35. doi: 10.1111/j.1365-2249.2006.03103.x. Clin Exp Immunol. 2006. PMID: 16792670 Free PMC article.
-
Autoantibodies to human alpha6 integrin in patients with oral pemphigoid.J Dent Res. 2001 Aug;80(8):1711-5. doi: 10.1177/00220345010800080601. J Dent Res. 2001. PMID: 11669480
-
Serological studies in bullous pemphigoid: a literature review of antibody titers at presentation and in clinical remission.Acta Derm Venereol. 2010 Mar;90(2):115-21. doi: 10.2340/00015555-0819. Acta Derm Venereol. 2010. PMID: 20169293 Review.
-
Immunodiagnosis of pemphigus and mucous membrane pemphigoid.Acta Odontol Scand. 2001 Aug;59(4):226-34. doi: 10.1080/00016350152509256. Acta Odontol Scand. 2001. PMID: 11570526 Review.
Cited by
-
Autoimmune bullous dermatoses in the elderly: diagnosis and management.Drugs Aging. 2003;20(9):663-81. doi: 10.2165/00002512-200320090-00004. Drugs Aging. 2003. PMID: 12831291 Review.
-
Reversing Autoimmunity Combination of Rituximab and Intravenous Immunoglobulin.Front Immunol. 2018 Jul 18;9:1189. doi: 10.3389/fimmu.2018.01189. eCollection 2018. Front Immunol. 2018. PMID: 30072982 Free PMC article.
-
Oral mucous membrane pemphigoid - Two case reports with varied clinical presentation.J Indian Soc Periodontol. 2016 Nov-Dec;20(6):630-634. doi: 10.4103/jisp.jisp_155_16. J Indian Soc Periodontol. 2016. PMID: 29238145 Free PMC article.
-
High-dose intravenous immunoglobulin (IVIG) therapy in autoimmune skin blistering diseases.Clin Rev Allergy Immunol. 2010 Apr;38(2-3):186-95. doi: 10.1007/s12016-009-8153-y. Clin Rev Allergy Immunol. 2010. PMID: 19557317 Review.
-
Autoimmune bullous dermatoses in the elderly: an update on pathophysiology, diagnosis and management.Drugs Aging. 2010 Jan 1;27(1):1-19. doi: 10.2165/11318600-000000000-00000. Drugs Aging. 2010. PMID: 20030429
References
-
- Mobini N, Nagarwalla N, Ahmed AR. Oral pemphigoid. Oral Surg Oral Med Oral Path Oral Radiol Endod. 1998;85:37–43. - PubMed
-
- Fleming TE, Korman NJ. Cicatricial Pemphigoid. J Am Acad Dermatol. 2000;43:571–91. - PubMed
-
- Laskaris G, Angelopoulos A. Cicatricial pemphigoid. direct and indirect immunofluorescence studies. Oral Surg Oral Med Oral Pathol. 1981;51:48–54. - PubMed
-
- Gammon WR, Briggaman RA, Inman A, Queen LL, Wheeler LE. Differentiating anti-lamina lucida and anti-sub lamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol. 1984;82:139–44. - PubMed
-
- Scully C, Carrozzo M, Gandolfo S, Puiatti P, Monteil R. Update on mucous membrane pemphigoid: a heterogeneous immune-mediated subepithelial entity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:56–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

