The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea
- PMID: 12198093
- PMCID: PMC2229520
- DOI: 10.1085/jgp.20028628
The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea
Abstract
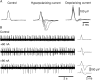
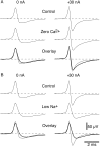
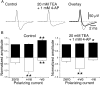
It was reported recently that action potentials actively invade the sensory nerve terminals of corneal polymodal receptors, whereas corneal cold receptor nerve terminals are passively invaded (Brock, J.A., S. Pianova, and C. Belmonte. 2001. J. Physiol. 533:493-501). The present study investigated whether this functional difference between these two types of receptor was due to an absence of voltage-activated Na(+) conductances in cold receptor nerve terminals. To address this question, the study examined the effects of polarizing current on the configuration of nerve terminal impulses recorded extracellularly from single polymodal and cold receptors in guinea-pig cornea isolated in vitro. Polarizing currents were applied through the recording electrode. In both receptor types, hyperpolarizing current (+ve) increased the negative amplitude of nerve terminal impulses. In contrast, depolarizing current (-ve) was without effect on polymodal receptor nerve terminal impulses but increased the positive amplitude of cold receptor nerve terminal impulses. The hyperpolarization-induced increase in the negative amplitude of nerve terminal impulses represents a net increase in inward current. In both types of receptor, this increase in inward current was reduced by local application of low Na(+) solution and blocked by lidocaine (10 mM). In addition, tetrodotoxin (1 microM) slowed but did not reduce the hyperpolarization-induced increase in the negative amplitude of polymodal and cold nerve terminal impulses. The depolarization-induced increase in the positive amplitude of cold receptor nerve terminal impulses represents a net increase in outward current. This change was reduced both by lidocaine (10 mM) and the combined application of tetraethylammomium (20 mM) and 4-aminopyridine (1 mM). The interpretation is that both polymodal and cold receptor nerve terminals possess high densities of tetrodotoxin-resistant Na(+) channels. This finding suggests that in cold receptors, under normal conditions, the Na(+) conductances are rendered inactive because the nerve terminal region is relatively depolarized.
Figures

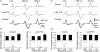
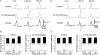
References
-
- Akopian, A.N., V. Souslova, S. England, K. Okuse, N. Ogata, J. Ure, A. Smith, B.J. Kerr, S.B. McMahon, S. Boyce, et al. 1999. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 2:541–548. - PubMed
-
- Botelho, S.Y., and E.V. Martinez. 1973. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am. J. Physiol. 225:606–609. - PubMed
-
- Belmonte, C., J. Garcia-Hirschfeld, and J. Gallar. 1997. Neurobiology of ocular pain. Prog. Retin. Eye Res. 16:117–156.

