A mitochondrial specific stress response in mammalian cells
- PMID: 12198143
- PMCID: PMC126185
- DOI: 10.1093/emboj/cdf445
A mitochondrial specific stress response in mammalian cells
Abstract
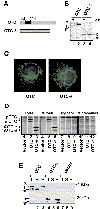
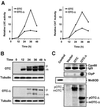
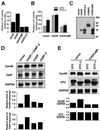
Cells respond to a wide variety of stresses through the transcriptional activation of genes that harbour stress elements within their promoters. While many of these elements are shared by genes encoding proteins representative of all subcellular compartments, cells can also respond to stresses that are specific to individual organelles, such as the endoplasmic reticulum un folded protein response. Here we report on the discovery and characterization of a mitochondrial stress response in mammalian cells. We find that the accumulation of unfolded protein within the mitochondrial matrix results in the transcriptional upregulation of nuclear genes encoding mitochondrial stress proteins such as chaperonin 60, chaperonin 10, mtDnaJ and ClpP, but not those encoding stress proteins of the endoplasmic reticulum. Analysis of the chaperonin 60/10 bidirectional promoter identified a CHOP element as the mitochondrial stress response element. Dominant-negative mutant forms of CHOP and overexpression of CHOP revealed that this transcription factor, in association with C/EBPbeta, regulates expression of mitochondrial stress genes in response to the accumulation of unfolded proteins.
Figures



References
-
- Bartlett J.D., Luethy,J.D., Carlson,S.G., Sollott,S.J. and Holbrook,N.J. (1992) Calcium ionophore A23187 induces expression of the growth arrest and DNA damage inducible CCAAT/enhancer-binding protein (C/EBP)-related gene, gadd153. Ca2+ increases transcriptional activity and mRNA stability. J. Biol. Chem., 267, 20465–20470. - PubMed
-
- Biswas G., Adebanjo,O.A., Freedman,B.D., Anandatheerthavarada,H.K., Vijayasarathy,C., Zaidi,M., Kotlikoff,M. and Avadhani,N.G. (1999) Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J., 18, 522–533. - PMC - PubMed
-
- Bruhat A., Jousse,C., Wang,X.Z., Ron,D., Ferrara,M. and Fafournoux,P. (1997) Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both tran scriptional and post-transcriptional levels. J. Biol. Chem., 272, 17588–17593. - PubMed
-
- Calfon M., Zeng,H., Urano,F., Till,J.H., Hubbard,S.R., Harding,H.P., Clark,S.G. and Ron,D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature, 415, 92–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

