Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library
- PMID: 12198145
- PMCID: PMC125414
- DOI: 10.1093/emboj/cdf474
Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library
Abstract
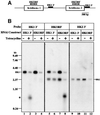
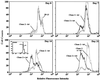
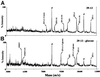
RNA interference (RNAi) is a powerful tool for identifying gene function in Trypanosoma brucei. We generated an RNAi library, the first of its kind in any organism, by ligation of genomic fragments into the vector pZJMbeta. After transfection at approximately 5-fold genome coverage, trypanosomes were induced to express double-stranded RNA and screened for reduced con canavalin A (conA) binding. Since this lectin binds the surface glycoprotein EP-procyclin, we predicted that cells would lose affinity to conA if RNAi silenced genes affecting EP-procyclin expression or modification. We found a cell line in which RNAi switches expression from glycosylated EP-procyclins to the unglycosylated GPEET-procyclin. This switch results from silencing a hexokinase gene. The relationship between procyclin expression and glycolysis was supported by silencing other genes in the glycolytic pathway, and confirmed by observation of a similar upregulation of GPEET- procyclin when parental cells were grown in medium depleted of glucose. These data suggest that T.brucei 'senses' changes in glucose level and modulates procyclin expression accordingly.
Figures




Similar articles
-
Killing of Trypanosoma brucei by concanavalin A: structural basis of resistance in glycosylation mutants.J Mol Biol. 2000 Dec 8;304(4):633-44. doi: 10.1006/jmbi.2000.4246. J Mol Biol. 2000. PMID: 11099385
-
'GPEET' procyclin is the major surface protein of procyclic culture forms of Trypanosoma brucei brucei strain 427.Biochem J. 1997 Sep 1;326 ( Pt 2)(Pt 2):415-23. doi: 10.1042/bj3260415. Biochem J. 1997. PMID: 9291113 Free PMC article.
-
RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei.Eukaryot Cell. 2003 Jun;2(3):542-51. doi: 10.1128/EC.2.3.542-551.2003. Eukaryot Cell. 2003. PMID: 12796299 Free PMC article.
-
Unravelling the procyclin coat of Trypanosoma brucei.Mol Biochem Parasitol. 1998 Mar 1;91(1):117-30. doi: 10.1016/s0166-6851(97)00195-3. Mol Biochem Parasitol. 1998. PMID: 9574930 Review. No abstract available.
-
RNA interference: advances and questions.Philos Trans R Soc Lond B Biol Sci. 2002 Jan 29;357(1417):65-70. doi: 10.1098/rstb.2001.0952. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 11839183 Free PMC article. Review.
Cited by
-
Mitochondrial shape and function in trypanosomes requires the outer membrane protein, TbLOK1.Mol Microbiol. 2013 Feb;87(4):713-29. doi: 10.1111/mmi.12089. Epub 2013 Jan 21. Mol Microbiol. 2013. PMID: 23336702 Free PMC article.
-
Expression of a major surface protein of Trypanosoma brucei insect forms is controlled by the activity of mitochondrial enzymes.Mol Biol Cell. 2004 Sep;15(9):3986-93. doi: 10.1091/mbc.e04-04-0341. Epub 2004 Jun 16. Mol Biol Cell. 2004. PMID: 15201340 Free PMC article.
-
High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome.Genome Res. 2011 Jun;21(6):915-24. doi: 10.1101/gr.115089.110. Epub 2011 Mar 1. Genome Res. 2011. PMID: 21363968 Free PMC article.
-
Glycolysis in the african trypanosome: targeting enzymes and their subcellular compartments for therapeutic development.Mol Biol Int. 2011;2011:123702. doi: 10.4061/2011/123702. Epub 2011 Apr 11. Mol Biol Int. 2011. PMID: 22091393 Free PMC article.
-
Isolation of the repertoire of VSG expression site containing telomeres of Trypanosoma brucei 427 using transformation-associated recombination in yeast.Genome Res. 2004 Nov;14(11):2319-29. doi: 10.1101/gr.2955304. Genome Res. 2004. PMID: 15520294 Free PMC article.
References
-
- Acosta-Serrano A., Cole,R.N., Mehlert,A., Lee,M.G., Ferguson,M.A. and Englund,P.T. (1999) The procyclin repertoire of Trypanosoma brucei. Identification and structural characterization of the Glu-Pro-rich polypeptides. J. Biol. Chem., 274, 29763–29771. - PubMed
-
- Acosta-Serrano A., Cole,R.N. and Englund,P.T. (2000) Killing of Trypanosoma brucei by concanavalin A: structural basis of resistance in glycosylation mutants. J. Mol. Biol., 304, 633–644. - PubMed
-
- Ashcroft F.M. and Gribble,F.M. (1999) ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia, 42, 903–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

