Vesicle transmembrane potential is required for translocation to the cytosol of externally added FGF-1
- PMID: 12198150
- PMCID: PMC126202
- DOI: 10.1093/emboj/cdf472
Vesicle transmembrane potential is required for translocation to the cytosol of externally added FGF-1
Abstract
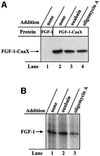
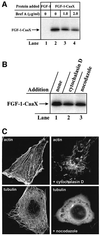
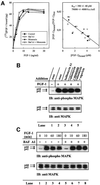
Externally added fibroblast growth factor-1 (FGF-1) is capable of crossing cellular membranes to reach the cytosol and the nucleus in a number of cell types. We have monitored the translocation of the growth factor by two methods: phosphorylation of FGF-1, and prenylation of an FGF-1 mutant that contains a C-terminal prenylation signal. Inhibition of endosomal acidification by ammonium chloride or monensin did not block the translocation of FGF-1, whereas bafilomycin A1, a specific inhibitor of vacuolar proton pumps, blocked translocation completely. A combination of ionophores expected to dissipate the vesicular membrane potential (valinomycin plus monensin) also fully inhibited the translocation. The inhibition of translocation by bafilomycin A1 was overcome in the presence of monensin or nigericin, while ouabain blocked translocation under these conditions. The data indicate that translocation of FGF-1 to cytosol occurs from the lumen of intracellular vesicles possessing vacuolar proton pumps, and that a vesicular membrane potential is required. Apparently, activation of vesicular Na+/K+-ATPase by monensin or nigericin generates a membrane potential that can support translocation when the proton pump is blocked.
Figures






Similar articles
-
Translocation of FGF-1 and FGF-2 across vesicular membranes occurs during G1-phase by a common mechanism.Mol Biol Cell. 2004 Feb;15(2):801-14. doi: 10.1091/mbc.e03-08-0589. Epub 2003 Dec 2. Mol Biol Cell. 2004. PMID: 14657241 Free PMC article.
-
FGF-1 and FGF-2 require the cytosolic chaperone Hsp90 for translocation into the cytosol and the cell nucleus.J Biol Chem. 2006 Apr 21;281(16):11405-12. doi: 10.1074/jbc.M600477200. Epub 2006 Feb 22. J Biol Chem. 2006. PMID: 16495214
-
An investigation on the role of vacuolar-type proton pumps and luminal acidity in calcium sequestration by nonmitochondrial and inositol-1,4,5-trisphosphate-sensitive intracellular calcium stores in clonal insulin-secreting cells.Eur J Biochem. 1994 Jun 15;222(3):869-77. doi: 10.1111/j.1432-1033.1994.tb18934.x. Eur J Biochem. 1994. PMID: 8026497
-
Evidence for vacuolar-type proton pumps in nonmitochondrial and inositol 1,4,5-trisphosphate-sensitive calcium stores of insulin-secreting cells.Pflugers Arch. 1996 May;432(1):97-104. doi: 10.1007/s004240050110. Pflugers Arch. 1996. PMID: 8662273
-
Energy coupling of L-glutamate transport and vacuolar H(+)-ATPase in brain synaptic vesicles.J Biochem. 1990 Oct;108(4):689-93. doi: 10.1093/oxfordjournals.jbchem.a123264. J Biochem. 1990. PMID: 2149857
Cited by
-
Phosphorylation of fibroblast growth factor (FGF) receptor 1 at Ser777 by p38 mitogen-activated protein kinase regulates translocation of exogenous FGF1 to the cytosol and nucleus.Mol Cell Biol. 2008 Jun;28(12):4129-41. doi: 10.1128/MCB.02117-07. Epub 2008 Apr 14. Mol Cell Biol. 2008. PMID: 18411303 Free PMC article.
-
Increased protein stability of FGF1 can compensate for its reduced affinity for heparin.J Biol Chem. 2009 Sep 11;284(37):25388-403. doi: 10.1074/jbc.M109.001289. Epub 2009 Jul 2. J Biol Chem. 2009. PMID: 19574212 Free PMC article.
-
Human rhinovirus type 2 uncoating at the plasma membrane is not affected by a pH gradient but is affected by the membrane potential.J Virol. 2009 Apr;83(8):3778-87. doi: 10.1128/JVI.01739-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193784 Free PMC article.
-
Cellular internalization of quantum dots noncovalently conjugated with arginine-rich cell-penetrating peptides.J Nanosci Nanotechnol. 2010 Oct;10(10):6534-43. doi: 10.1166/jnn.2010.2637. J Nanosci Nanotechnol. 2010. PMID: 21137758 Free PMC article.
-
Avian infectious bronchitis virus enters cells via the endocytic pathway.Adv Exp Med Biol. 2006;581:309-12. doi: 10.1007/978-0-387-33012-9_54. Adv Exp Med Biol. 2006. PMID: 17037550 Free PMC article. No abstract available.
References
-
- Agarraberes F.A. and Dice,J.F. (2001) Protein translocation across membranes. Biochim. Biophys. Acta, 1513, 1–24. - PubMed
-
- Bomsel M., Parton,R., Kuznetsov,S.A., Schroer,T.A. and Gruenberg,J. (1990) Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell, 62, 719–731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

