An inositol 1,4,5-trisphosphate receptor-dependent cation entry pathway in DT40 B lymphocytes
- PMID: 12198155
- PMCID: PMC126200
- DOI: 10.1093/emboj/cdf467
An inositol 1,4,5-trisphosphate receptor-dependent cation entry pathway in DT40 B lymphocytes
Abstract
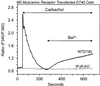
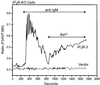
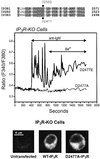
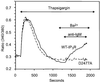
We examined the roles of inositol 1,4,5-trisphosphate (IP3) receptors (IP3R) in calcium signaling using DT40 B lymphocytes, and a variant lacking the three IP3R isoforms (IP3R-KO). In wild-type cells, B cell receptor (BCR) stimulation activates a cation entry route that exhibits significantly greater permeability to Ba2+ than does capacitative calcium entry. This cation entry is absent in IP3R-KO cells. Expression of the type-3 IP3R (IP3R-3) in the IP3R-KO cells rescued not only agonist-dependent release of intracellular Ca2+, but also Ba2+ influx following receptor stimulation. Similar results were obtained with an IP3R-3 mutant carrying a conservative point mutation in the selectivity filter region of the channel (D2477E); however, an IP3R-3 mutant in which this same aspartate was replaced by alanine (D2477A) failed to restore either BCR-induced Ca2+ release or receptor-dependent Ba2+ entry. These results suggest that in DT40 B lymphocytes, BCR stimulation activates a novel cation entry across the plasma membrane that depends upon, or is mediated by, fully functional IP3R.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

