A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium
- PMID: 12198158
- PMCID: PMC126179
- DOI: 10.1093/emboj/cdf438
A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium
Abstract
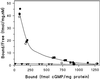
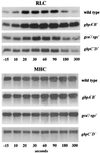
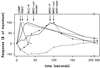
Chemotactic stimulation of Dictyostelium cells results in a transient increase in cGMP levels, and transient phosphorylation of myosin II heavy and regulatory light chains. In Dictyostelium, two guanylyl cyclases and four candidate cGMP-binding proteins (GbpA- GbpD) are implicated in cGMP signalling. GbpA and GbpB are homologous proteins with a Zn2+-hydrolase domain. A double gbpA/gbpB gene disruption leads to a reduction of cGMP-phosphodiesterase activity and a 10-fold increase of basal and stimulated cGMP levels. Chemotaxis in gbpA(-)B(-) cells is associated with increased myosin II phosphorylation compared with wild-type cells; formation of lateral pseudopodia is suppressed resulting in enhanced chemotaxis. GbpC is homologous to GbpD, and contains Ras, MAPKKK and Ras-GEF domains. Inactivation of the gbp genes indicates that only GbpC harbours high affinity cGMP-binding activity. Myosin phosphorylation, assembly of myosin in the cytoskeleton as well as chemotaxis are severely impaired in mutants lacking GbpC and GbpD, or mutants lacking both guanylyl cyclases. Thus, a novel cGMP signalling cascade is critical for chemotaxis in Dictyostelium, and plays a major role in myosin II regulation during this process.
Figures


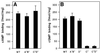


References
-
- Berlot C.H., Devreotes,P.N. and Spudich,J.A. (1987) Chemoattractant-elicited increases in Dictyostelium myosin phosphorylation are due to changes in myosin localization and increases in kinase activity. J. Biol. Chem., 262, 3918–3926. - PubMed
-
- Boy-Marcotte E. and Jacquet,M. (1982) A Dictyostelium discoideum DNA fragment complements a Saccharomyces cerevisiae ura3 mutant. Gene, 20, 433–439. - PubMed
-
- Chandrasekhar A., Wessels,D. and Soll,D.R. (1995) A mutation that depresses cGMP phosphodiesterase activity in Dictyostelium affects cell motility through an altered chemotactic signal. Dev. Biol., 169, 109–122. - PubMed
-
- de la Roche M.A. and Cote,G.P. (2001) Regulation of Dictyostelium myosin I and II. Biochim. Biophys. Acta, 1525, 245–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

